Abstract
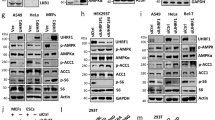
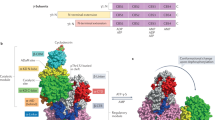
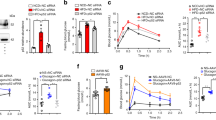
Liver kinase B1 (LKB1) has important roles in governing energy homeostasis by regulating the activity of the energy sensor kinase AMP-activated protein kinase (AMPK). The regulation of LKB1 function, however, is still poorly understood. Here we demonstrate that the orphan nuclear receptor Nur77 binds and sequesters LKB1 in the nucleus, thereby attenuating AMPK activation. This Nur77 function is antagonized by the chemical compound ethyl 2-[2,3,4-trimethoxy-6-(1-octanoyl)phenyl]acetate (TMPA), which interacts with Nur77 with high affinity and at specific sites. TMPA binding of Nur77 results in the release and shuttling of LKB1 to the cytoplasm to phosphorylate AMPKα. Moreover, TMPA effectively reduces blood glucose and alleviates insulin resistance in type II db/db and high-fat diet– and streptozotocin-induced diabetic mice but not in diabetic littermates with the Nur77 gene knocked out. This study attains a mechanistic understanding of the regulation of LKB1-AMPK axis and implicates Nur77 as a new and amenable target for the design and development of therapeutics to treat metabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
28 September 2012
In the version of this article initially published online, the name of the contributing author Chawnshang Chang was misspelled. The error has been corrected for the PDF and HTML versions of this article.
References
Hardie, D.G. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 25, 1895–1908 (2011).
Munday, M.R., Campbell, D.G., Carling, D. & Hardie, D.G. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur. J. Biochem. 175, 331–338 (1988).
Koo, S.H. et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 (2005).
Sambandam, N. & Lopaschuk, G.D. AMP-activated protein kinase (AMPK) control of fatty acid and glucose metabolism in the ischemic heart. Prog. Lipid Res. 42, 238–256 (2003).
Winder, W.W. et al. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J. Appl. Physiol. 82, 219–225 (1997).
Minokoshi, Y. et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415, 339–343 (2002).
Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 (2002).
Kishi, K. et al. AMP-activated protein kinase is activated by the stimulations of Gq-coupled receptors. Biochem. Biophys. Res. Commun. 276, 16–22 (2000).
Huypens, P., Quartier, E., Pipeleers, D. & Van de Casteele, M. Metformin reduces adiponectin protein expression and release in 3T3-L1 adipocytes involving activation of AMP activated protein kinase. Eur. J. Pharmacol. 518, 90–95 (2005).
Hardie, D.G. & Hawley, S.A. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays 23, 1112–1119 (2001).
Oakhill, J.S. et al. β-subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc. Natl. Acad. Sci. USA 107, 19237–19241 (2010).
Suter, M. et al. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 281, 32207–32216 (2006).
Hawley, S.A. et al. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2, 28 (2003).
Shaw, R.J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005).
Hawley, S.A. et al. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 (2005).
Herrero-Martín, G. et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 28, 677–685 (2009).
Kim, E.J. et al. RORα-induced activation of AMP-activated protein kinase results in attenuation of hepatic steatosis. Hepatology 55, 1379–1388 (2012).
Lee, C.G. et al. Farnesoid X receptor protects hepatocytes from injury by repressing miR-199a-3p, which increases levels of LKB1. Gastroenterology 142, 1206–1217 e7 (2012).
Linher-Melville, K., Zantinge, S. & Singh, G. Liver kinase B1 expression (LKB1) is repressed by estrogen receptor α (ERα) in MCF-7 human breast cancer cells. Biochem. Biophys. Res. Commun. 417, 1063–1068 (2012).
Nath-Sain, S. & Marignani, P.A. LKB1 catalytic activity contributes to estrogen receptor α signaling. Mol. Biol. Cell 20, 2785–2795 (2009).
Germain, P., Staels, B., Dacquet, C., Spedding, M. & Laudet, V. Overview of nomenclature of nuclear receptors. Pharmacol. Rev. 58, 685–704 (2006).
Fu, Y., Luo, L., Luo, N., Zhu, X. & Garvey, W.T. NR4A orphan nuclear receptors modulate insulin action and the glucose transport system: potential role in insulin resistance. J. Biol. Chem. 282, 31525–31533 (2007).
Chao, L.C. et al. Nur77 coordinately regulates expression of genes linked to glucose metabolism in skeletal muscle. Mol. Endocrinol. 21, 2152–2163 (2007).
Pei, L. et al. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat. Med. 12, 1048–1055 (2006).
Chao, L.C. et al. Insulin resistance and altered systemic glucose metabolism in mice lacking Nur77. Diabetes 58, 2788–2796 (2009).
Zhan, Y. et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat. Chem. Biol. 4, 548–556 (2008).
Crute, B.E., Seefeld, K., Gamble, J., Kemp, B.E. & Witters, L.A. Functional domains of the α1 catalytic subunit of the AMP-activated protein kinase. J. Biol. Chem. 273, 35347–35354 (1998).
Li, Y. et al. Molecular recognition of nitrated fatty acids by PPARγ. Nat. Struct. Mol. Biol. 15, 865–867 (2008).
Pike, A.C. et al. Structure of the ligand-binding domain of oestrogen receptor β in the presence of a partial agonist and a full antagonist. EMBO J. 18, 4608–4618 (1999).
Renaud, J.P. et al. Crystal structure of the RAR-γ ligand-binding domain bound to all-trans retinoic acid. Nature 378, 681–689 (1995).
Shiau, A.K. et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95, 927–937 (1998).
Baas, A.F. et al. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 22, 3062–3072 (2003).
Xie, Z., Dong, Y., Scholz, R., Neumann, D. & Zou, M.H. Phosphorylation of LKB1 at serine 428 by protein kinase C-ζ is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 117, 952–962 (2008).
Karuman, P. et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol. Cell 7, 1307–1319 (2001).
Xie, Z. et al. Identification of the serine 307 of LKB1 as a novel phosphorylation site essential for its nucleocytoplasmic transport and endothelial cell angiogenesis. Mol. Cell. Biol. 29, 3582–3596 (2009).
Tiainen, M., Vaahtomeri, K., Ylikorkala, A. & Makela, T.P. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1). Hum. Mol. Genet. 11, 1497–1504 (2002).
Boudeau, J. et al. MO25α/β interact with STRADα/β enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 22, 5102–5114 (2003).
Sakamoto, K., Goransson, O., Hardie, D.G. & Alessi, D.R. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am. J. Physiol. Endocrinol. Metab. 287, E310–E317 (2004).
Viollet, B. et al. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J. Physiol. (Lond.) 574, 41–53 (2006).
Coleman, D.L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14, 141–148 (1978).
Srinivasan, K., Viswanad, B., Asrat, L., Kaul, C.L. & Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 52, 313–320 (2005).
Gilbert, E.R., Fu, Z. & Liu, D. Development of a nongenetic mouse model of type 2 diabetes. Exp. Diabetes Res. 2011, 416254 (2011).
Deepa, S.S. et al. APPL1 mediates adiponectin-induced LKB1 cytosolic localization through the PP2A-PKCγ signaling pathway. Mol. Endocrinol. 25, 1773–1785 (2011).
Kimball, S.R., Siegfried, B.A. & Jefferson, L.S. Glucagon represses signaling through the mammalian target of rapamycin in rat liver by activating AMP-activated protein kinase. J. Biol. Chem. 279, 54103–54109 (2004).
Acknowledgements
We are grateful to P. Li (Tsinghua University) for the plasmids encoding AMPKβ, AMPKγ and AMPKα(1–312) and to J. Wu (Tsinghua University) for the tricistronic expression plasmids for all three subunits of AMPK. This work was supported by the grants from '973' Project of the Ministry of Science and Technology (2011CB910802), the National Natural Science Fund of China (30810103905, 30630070, 30921005 and 30870479), the Program of Introducing Talents of Discipline to Universities (B12001 and B06016) and the Open Research Fund of State Key Laboratory of Cellular Stress Biology, Xiamen University (SKLCSB2012KF002). We also gratefully acknowledge the use of Beamline BL17U1 at Shanghai Synchrotron Radiation Facility for crystallographic data collection.
Author information
Authors and Affiliations
Contributions
Q.W. and T.L designed the experiments and wrote the manuscript. Y.Z., Y.C., M.T., H.C., J.H., W.W., R.W., Y.W., A.L., Y.X. and T.Y.L. carried out the experiments on molecular and cellular biology as well as studies on mouse models. Q.Z., L.Z., C.S. and K.Y. carried out the structural studies. J.Z. and H.Z. synthesized the chemical compounds, and S.-C.L., P.H., C.C., J.Y.Y., Z.Z. and C.Y. were involved in the design of this project as well as in reading and commenting on the manuscript, and S.-C.L. helped on writing the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 1258 kb)
Rights and permissions
About this article
Cite this article
Zhan, Yy., Chen, Y., Zhang, Q. et al. The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK. Nat Chem Biol 8, 897–904 (2012). https://doi.org/10.1038/nchembio.1069
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1069
This article is cited by
-
Mannose antagonizes GSDME-mediated pyroptosis through AMPK activated by metabolite GlcNAc-6P
Cell Research (2023)
-
Time-of-day defines NAD+ efficacy to treat diet-induced metabolic disease by synchronizing the hepatic clock in mice
Nature Communications (2023)
-
Long non-coding RNA SNHG6 couples cholesterol sensing with mTORC1 activation in hepatocellular carcinoma
Nature Metabolism (2022)
-
mPGES-2 blockade antagonizes β-cell senescence to ameliorate diabetes by acting on NR4A1
Nature Metabolism (2022)
-
Prostaglandin A2 Interacts with Nurr1 and Ameliorates Behavioral Deficits in Parkinson’s Disease Fly Model
NeuroMolecular Medicine (2022)