Abstract
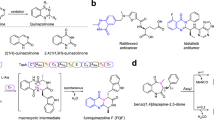
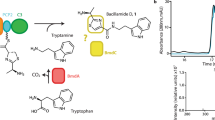
Cyclization of linear peptidyl precursors produced by nonribosomal peptide synthetases (NRPSs) is an important step in the biosynthesis of bioactive cyclic peptides. Whereas bacterial NRPSs use thioesterase domains to perform the cyclization, fungal NRPSs have apparently evolved to use a different enzymatic route. In verified fungal NRPSs that produce macrocyclic peptides, each megasynthetase terminates with a condensation-like (CT) domain that may perform the macrocyclization reaction. To probe the role of such a CT domain, we reconstituted the activities of the Penicillium aethiopicum trimodular NPRS TqaA in Saccharomyces cerevisiae and in vitro. Together with the reconstituted bimodular NRPS AnaPS, we dissected the cyclization steps of TqaA in transforming the linear anthranilate-D-tryptophan-L-alanyl tripeptide into fumiquinazoline F. Extensive biochemical and mutational studies confirmed the essential role of the CT domain in catalyzing cyclization in a thiolation domain–dependent fashion. Our work provides evidence of a likely universal macrocyclization strategy used by fungal NRPSs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bierer, B.E., Hollander, G., Fruman, D. & Burakoff, S.J. Cyclosporine A and FK506: molecular mechanisms of immunosuppression and probes for transplantation biology. Curr. Opin. Immunol. 5, 763–773 (1993).
Chen, S.C., Slavin, M.A. & Sorrell, T.C. Echinocandin antifungal drugs in fungal infections: a comparison. Drugs 71, 11–41 (2011).
Sieber, S.A. & Marahiel, M.A. Molecular mechanisms underlying nonribosomal peptide synthesis: approaches to new antibiotics. Chem. Rev. 105, 715–738 (2005).
Sattely, E.S., Fischbach, M.A. & Walsh, C.T. Total biosynthesis: in vitro reconstitution of polyketide and nonribosomal peptide pathways. Nat. Prod. Rep. 25, 757–793 (2008).
Fischbach, M.A. & Walsh, C.T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem. Rev. 106, 3468–3496 (2006).
Kohli, R.M., Walsh, C.T. & Burkart, M.D. Biomimetic synthesis and optimization of cyclic peptide antibiotics. Nature 418, 658–661 (2002).
Kopp, F. & Marahiel, M.A. Macrocyclization strategies in polyketide and nonribosomal peptide biosynthesis. Nat. Prod. Rep. 24, 735–749 (2007).
Du, L. & Lou, L.L. PKS and NRPS release mechanisms. Nat. Prod. Rep. 27, 255–278 (2010).
von Döhren, H. A survey of nonribosomal peptide synthetase (NRPS) genes in Aspergillus nidulans. Fungal Genet. Biol. 46, S45–S52 (2009).
Eisfeld, K. Non-Ribosomal Peptide Synthetases of Fungi. in Physiology and Genetics 1st edn., Vol. 15 (eds. Anke, T. & Weber, D.) 315–316 (Springer-Verlag, Berlin Heidelberg, 2009).
Lawen, A. & Zocher, R. Cyclosporin synthetase. The most complex peptide synthesizing multienzyme polypeptide so far described. J. Biol. Chem. 265, 11355–11360 (1990).
Jin, J.M. et al. Functional characterization and manipulation of the apicidin biosynthetic pathway in Fusarium semitectum. Mol. Microbiol. 76, 456–466 (2010).
Slightom, J.L., Metzger, B.R., Luu, H.T. & Elhammer, A.P. Cloning and molecular characterization of the gene encoding the aureobasidin A biosynthesis complex in Aureobasidium pullulans BP-1938. Gene 431, 67–79 (2009).
Winterberg, B. et al. Elucidation of the complete ferrichrome A biosynthetic pathway in Ustilago maydis. Mol. Microbiol. 75, 1260–1271 (2010).
Zocher, R. et al. Biosynthesis of cyclosporin A: partial purification and properties of a multifunctional enzyme from Tolypocladium inflatum. Biochemistry 25, 550–553 (1986).
Gao, X. et al. Fungal indole alkaloid biosynthesis: genetic and biochemical investigation of the tryptoquialanine pathway in Penicillium aethiopicum. J. Am. Chem. Soc. 133, 2729–2741 (2011).
Bergendahl, V., Linne, U. & Marahiel, M.A. Mutational analysis of the C-domain in nonribosomal peptide synthesis. Eur. J. Biochem. 269, 620–629 (2002).
Keating, T.A., Marshall, C.G., Walsh, C.T. & Keating, A.E. The structure of VibH represents nonribosomal peptide synthetase condensation, cyclization and epimerization domains. Nat. Struct. Biol. 9, 522–526 (2002).
Samel, S.A., Schoenafinger, G., Knappe, T.A., Marahiel, M.A. & Essen, L.O. Structural and functional insights into a peptide bond–forming bidomain from a nonribosomal peptide synthetase. Structure 15, 781–792 (2007).
Ames, B.D. & Walsh, C.T. Anthranilate-activating modules from fungal nonribosomal peptide assembly lines. Biochemistry 49, 3351–3365 (2010).
Ames, B.D., Liu, X. & Walsh, C.T. Enzymatic processing of fumiquinazoline F: a tandem oxidative-acylation strategy for the generation of multicyclic scaffolds in fungal indole alkaloid biosynthesis. Biochemistry 49, 8564–8576 (2010).
Yin, W.B., Grundmann, A., Cheng, J. & Li, S.M. Acetylaszonalenin biosynthesis in Neosartorya fischeri. Identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J. Biol. Chem. 284, 100–109 (2009).
Shao, Z., Zhao, H. & Zhao, H. DNA assembler, an in vivo genetic method for rapid construction of biochemical pathways. Nucleic Acids Res. 37, e16 (2009).
Ma, S.M. et al. Complete reconstitution of a highly reducing iterative polyketide synthase. Science 326, 589–592 (2009).
Mootz, H.D., Schorgendorfer, K. & Marahiel, M.A. Functional characterization of 4′ phosphopantetheinyl transferase genes of bacterial and fungal origin by complementation of Saccharomyces cerevisiae lys5. FEMS Microbiol. Lett. 213, 51–57 (2002).
Xu, W., Cai, X.L., Jung, M.E. & Tang, Y. Analysis of intact and dissected fungal polyketide synthase-nonribosomal peptide synthetase in vitro and in Saccharomyces cerevisiae. J. Am. Chem. Soc. 132, 13604–13607 (2010).
Rusnak, F., Sakaitani, M., Drueckhammer, D., Reichert, J. & Walsh, C.T. Biosynthesis of the Escherichia coli siderophore enterobactin: sequence of the entf gene, expression and purification of Entf, and analysis of covalent phosphopantetheine. Biochemistry 30, 2916–2927 (1991).
Stachelhaus, T. & Walsh, C.T. Mutational analysis of the epimerization domain in the initiation module PheATE of gramicidin S synthetase. Biochemistry 39, 5775–5787 (2000).
Linne, U. & Marahiel, M.A. Control of directionality in nonribosomal peptide synthesis: role of the condensation domain in preventing misinitiation and timing of epimerization. Biochemistry 39, 10439–10447 (2000).
Sieber, S.A., Tao, J.H., Walsh, C.T. & Marahiel, M.A. Peptidyl thiophenols as substrates for nonribosomal peptide cyclases. Angew. Chem. Int. Edn Engl. 43, 493–498 (2004).
Quadri, L.E.N. et al. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 37, 1585–1595 (1998).
Xie, X., Meehan, M.J., Xu, W., Dorrestein, P.C. & Tang, Y. Acyltransferase mediated polyketide release from a fungal megasynthase. J. Am. Chem. Soc. 131, 8388–8389 (2009).
Balibar, C.J. & Walsh, C.T. GliP, a multimodular nonribosomal peptide synthetase in Aspergillus fumigatus, makes the diketopiperazine scaffold of gliotoxin. Biochemistry 45, 15029–15038 (2006).
Keating, T.A., Miller, D.A. & Walsh, C.T. Expression, purification, and characterization of HMWP2, a 229 kDa, six domain protein subunit of yersiniabactin synthetase. Biochemistry 39, 4729–4739 (2000).
Lee, J.H., Evans, B.S., Li, G.Y., Kelleher, N.L. & van der Donk, W.A. In vitro characterization of a heterologously expressed nonribosomal peptide synthetase involved in phosphinothricin tripeptide biosynthesis. Biochemistry 48, 5054–5056 (2009).
Ames, B.D. et al. Complexity generation in fungal peptidyl alkaloid biosynthesis: oxidation of fumiquinazoline A to the heptacyclic hemiaminal fumiquinazoline C by the flavoenzyme Af12070 from Aspergillus fumigatus. Biochemistry 50, 8756–8769 (2011).
Daniel, J.F.D. & Rodrigues, E. Peptaibols of Trichoderma. Nat. Prod. Rep. 24, 1128–1141 (2007).
Liu, X. & Walsh, C.T. Cyclopiazonic acid biosynthesis in Aspergillus sp.: characterization of a reductase-like R* domain in cyclopiazonate synthetase that forms and releases cyclo-acetoacetyl-L-tryptophan. Biochemistry 48, 8746–8757 (2009).
Sims, J.W. & Schmidt, E.W. Thioesterase-like role for fungal PKS-NRPS hybrid reductive domains. J. Am. Chem. Soc. 130, 11149–11155 (2008).
Wang, B., Kang, Q., Lu, Y., Bai, L. & Wang, C. Unveiling the biosynthetic puzzle of destruxins in Metarhizium species. Proc. Natl. Acad. Sci. USA 109, 1287–1292 (2012).
Grindberg, R.V. et al. Single cell genome amplification accelerates identification of the apratoxin biosynthetic pathway from a complex microbial assemblage. PLoS ONE 6, e18565 (2011).
Gatto, G.J., McLoughlin, S.M., Kelleher, N.L. & Walsh, C.T. Elucidating the substrate specificity and condensation domain activity of FkbP, the FK520 pipecolate-incorporating enzyme. Biochemistry 44, 5993–6002 (2005).
Schwecke, T. et al. The biosynthetic gene-cluster for the polyketide immunosuppressant rapamycin. Proc. Natl. Acad. Sci. USA 92, 7839–7843 (1995).
Sieber, S.A., Walsh, C.T. & Marahiel, M.A. Loading peptidyl-coenzyme A onto peptidyl carrier proteins: a novel approach in characterizing macrocyclization by thioesterase domains. J. Am. Chem. Soc. 125, 10862–10866 (2003).
Acknowledgements
This work is supported in part by the US National Institutes of Health Grant GM20011 (to C.T.W.), F32GM090475 (to B.D.A.) and 1R01GM092217 (to Y.T.). W. Xu (University of California–Los Angeles) is thanked for assistance with MALDI-TOF mass analysis and for providing both the ApdA and LovF ACP domains. Y.-T. Lai (University of California–Los Angeles) is thanked for assistance with gel filtration FPLC.
Author information
Authors and Affiliations
Contributions
X.G., S.W.H., C.T.W. and Y.T. developed the hypothesis and designed the study. S.W.H. performed the synthesis of all of the chemicals in this study. X.G. and P.W. performed molecular cloning. X.G., P.W. and L.P.V. performed the heterologous protein expression and purification. X.G. and B.D.A. performed in vitro and in vivo characterization of the megasynthases. All authors analyzed and discussed the results. X.G., C.T.W. and Y.T. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 2897 kb)
Rights and permissions
About this article
Cite this article
Gao, X., Haynes, S., Ames, B. et al. Cyclization of fungal nonribosomal peptides by a terminal condensation-like domain. Nat Chem Biol 8, 823–830 (2012). https://doi.org/10.1038/nchembio.1047
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1047
This article is cited by
-
Three-membered ring formation catalyzed by α-ketoglutarate-dependent nonheme iron enzymes
Journal of Natural Medicines (2024)
-
A genetic tool to express long fungal biosynthetic genes
Fungal Biology and Biotechnology (2023)
-
Elucidating the molecular programming of a nonlinear non-ribosomal peptide synthetase responsible for fungal siderophore biosynthesis
Nature Communications (2023)
-
Commensal production of a broad-spectrum and short-lived antimicrobial peptide polyene eliminates nasal Staphylococcus aureus
Nature Microbiology (2023)
-
Efficient production of a cyclic dipeptide (cyclo-TA) using heterologous expression system of filamentous fungus Aspergillus oryzae
Microbial Cell Factories (2022)