Abstract
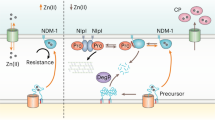
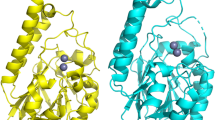
A number of multiresistant bacterial pathogens inactivate antibiotics by producing Zn(II)-dependent β-lactamases. We show that metal uptake leading to an active dinuclear enzyme in the periplasmic space of Gram-negative bacteria is ensured by a cysteine residue, an unusual metal ligand in oxidizing environments. Kinetic, structural and affinity data show that such Zn(II)-cysteine interaction is an adaptive trait that tunes the metal binding affinity, thus enabling antibiotic resistance at restrictive Zn(II) concentrations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Crowder, M.W., Spencer, J. & Vila, A.J. Acc. Chem. Res. 39, 721–728 (2006).
Fisher, J.F., Meroueh, S.O. & Mobashery, S. Chem. Rev. 105, 395–424 (2005).
Fabiane, S.M. et al. Biochemistry 37, 12404–12411 (1998).
Orellano, E.G., Girardini, J.E., Cricco, J.A., Ceccarelli, E.A. & Vila, A.J. Biochemistry 37, 10173–10180 (1998).
Hu, Z., Peryannan, G., Bennett, B. & Crowder, M. J. Am. Chem. Soc. 130, 14207–14216 (2008).
Hawk, M.J. et al. J. Am. Chem. Soc. 131, 10753–10762 (2009).
Llarrull, L.I., Tioni, M.F. & Vila, A.J. J. Am. Chem. Soc. 130, 15842–15851 (2008).
Wommer, S. et al. J. Biol. Chem. 277, 24142–24147 (2002).
Tioni, M.F. et al. J. Am. Chem. Soc. 130, 15852–15863 (2008).
Badarau, A. & Page, M.I. Biochemistry 45, 11012–11020 (2006).
Carfi, A. et al. EMBO J. 14, 4914–4921 (1995).
Murphy, T.A. et al. J. Mol. Biol. 357, 890–903 (2006).
González, J.M., Buschiazzo, A. & Vila, A.J. Biochemistry 49, 7930–7938 (2010).
Davis, A.V. & O'Halloran, T.V. Nat. Chem. Biol. 4, 148–151 (2008).
Daiyasu, H., Osaka, K., Ishino, Y. & Toh, H. FEBS Lett. 503, 1–6 (2001).
Haruta, S. et al. Antimicrob. Agents Chemother. 44, 2304–2309 (2000).
Llarrull, L.I., Tioni, M.F., Kowalski, J., Bennett, B. & Vila, A.J. J. Biol. Chem. 282, 30586–30595 (2007).
Rasia, R.M. & Vila, A.J. J. Biol. Chem. 279, 26046–26051 (2004).
Simona, F. et al. J. Biol. Chem. 284, 28164–28171 (2009).
Jacquin, O. et al. J. Mol. Biol. 392, 1278–1291 (2009).
Outten, C.E. & O'Halloran, T.V. Science 292, 2488–2492 (2001).
Ma, Z., Jacobsen, F.E. & Giedroc, D.P. Chem. Rev. 109, 4644–4681 (2009).
Morán-Barrio, J., Limansky, A.S. & Viale, A.M. Antimicrob. Agents Chemother. 53, 2908–2917 (2009).
Acknowledgements
This work has been supported by CONICET and by grants from the Howard Hughes Medical Institute, Agencia Nacional de Promoción Científica y Tecnológica, US National Institutes of Health (1R01AI100560 to A.J.V.) and Laboratório Nacional de Luz Síncrotron, Campinas, Brazil. A.J.V. is a fellow of the John Simon Guggenheim Foundation.
Author information
Authors and Affiliations
Contributions
J.M.G., J.A.C., M.-R.M., P.E.T. and A.J.V. designed experiments and analyzed results. J.M.G., M.-R.M., P.E.T. and A.J.V. wrote the manuscript. J.M.G., J.A.C. and M.-R.M. expressed and purified proteins. J.M.G. and J.A.C. determined kinetic parameters and performed cobalt substitution. J.M.G. performed stopped-flow measurements. M.-R.M. determined the dissociation constants for Zn(II) by competition experiments and the activity dependence on Zn(II) concentration. J.M.G. and F.J.M.M. determined the crystal structures. J.A.C. designed and made plasmid constructs, determined minimum inhibitory concentrations and performed in vivo antibiotic sensitivity tests. J.A.C. and M.-R.M. performed periplasmic extracts and western blot assays.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 945 kb)
Rights and permissions
About this article
Cite this article
González, J., Meini, MR., Tomatis, P. et al. Metallo-β-lactamases withstand low Zn(II) conditions by tuning metal-ligand interactions. Nat Chem Biol 8, 698–700 (2012). https://doi.org/10.1038/nchembio.1005
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1005
This article is cited by
-
In-cell kinetic stability is an essential trait in metallo-β-lactamase evolution
Nature Chemical Biology (2023)
-
The antimicrobial peptide thanatin disrupts the bacterial outer membrane and inactivates the NDM-1 metallo-β-lactamase
Nature Communications (2019)
-
A general reaction mechanism for carbapenem hydrolysis by mononuclear and binuclear metallo-β-lactamases
Nature Communications (2017)
-
Membrane anchoring stabilizes and favors secretion of New Delhi metallo-β-lactamase
Nature Chemical Biology (2016)