Abstract
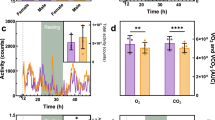
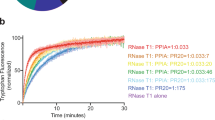
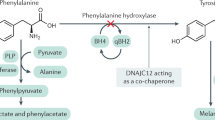
Phenylketonuria (PKU) is characterized by phenylalanine accumulation and progressive mental retardation caused by an unknown mechanism. We demonstrate that at pathological concentrations, phenylalanine self-assembles into fibrils with amyloid-like morphology and well-ordered electron diffraction. These assemblies are specifically recognized by antibodies, show cytotoxicity that can be neutralized by the antibodies and are present in the hippocampus of model mice and in parietal cortex brain tissue from individuals with PKU. This is, to our knowledge, the first demonstration that a single amino acid can form amyloid-like deposits, suggesting a new amyloidosis-like etiology for PKU.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hanley, W.B. Adult phenylketonuria. Am. J. Med. 117, 590–595 (2004).
Surtees, R. & Blau, N. The neurochemistry of phenylketonuria. Eur. J. Pediatr. 159 (suppl.), S109–S113 (2000).
Choi, T.B. & Pardridge, W.M. Phenylalanine transport at the human blood-brain barrier. Studies with isolated human brain capillaries. J. Biol. Chem. 261, 6536–6541 (1986).
Krause, W. et al. Biochemical and neuropsychological effects of elevated plasma phenylalanine in patients with treated phenylketonuria. A model for the study of phenylalanine and brain function in man. J. Clin. Invest. 75, 40–48 (1985).
MacDonald, A., Gokmen-Ozel, H., van Rijn, M. & Burgard, P. The reality of dietary compliance in the management of phenylketonuria. J. Inherit. Metab. Dis. 33, 665–670 (2010).
Chiti, F. & Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 (2006).
Rochet, J.C. & Lansbury, P.T. Amyloid fibrillogenesis: themes and variations. Curr. Opin. Struct. Biol. 10, 60–68 (2000).
Inouye, H., Sharma, D., Goux, W.J. & Kirschner, D.A. Structure of core domain of fibril-forming PHF/Tau fragments. Biophys. J. 90, 1774–1789 (2006).
Gazit, E. A possible role for π-stacking in the self-assembly of amyloid fibrils. FASEB J. 16, 77–83 (2002).
Makin, O.S. & Serpell, L.C. Structures for amyloid fibrils. FEBS J. 272, 5950–5961 (2005).
Gazit, E. Global analysis of tandem aromatic octapeptide repeats: the significance of the aromatic-glycine motif. Bioinformatics 18, 880–883 (2002).
Reches, M., Porat, Y. & Gazit, E. Amyloid fibril formation by pentapeptide and tetrapeptide fragments of human calcitonin. J. Biol. Chem. 277, 35475–35480 (2002).
Reches, M. & Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 300, 625–627 (2003).
Tjernberg, L.O. et al. Arrest of β-amyloid fibril formation by a pentapeptide ligand. J. Biol. Chem. 271, 8545–8548 (1996).
Soto, C. β-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer's therapy. Nat. Med. 4, 822–826 (1998).
Hörster, F. et al. Phenylalanine reduces synaptic density in mixed cortical cultures from mice. Pediatr. Res. 59, 544–548 (2006).
Makin, O.S., Atkins, E., Sikorski, P., Johansson, J. & Serpell, L.C. Molecular basis for amyloid fibril formation and stability. Proc. Natl. Acad. Sci. USA 102, 315–320 (2005).
Brooks, B.R. et al. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 (2009).
Haberthür, U. & Caflisch, A. FACTS: fast analytical continuum treatment of solvation. J. Comput. Chem. 29, 701–715 (2008).
Lesné, S. et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 (2006).
Abramov, E. et al. Amyloid-β as a positive endogenous regulator of release probability at hippocampal synapses. Nat. Neurosci. 12, 1567–1576 (2009).
Shedlovsky, A., McDonald, J.D., Symula, D. & Dove, W.F. Mouse models of human phenylketonuria. Genetics 134, 1205–1210 (1993).
Gazit, V., Ben-Abraham, R., Pick, C.G. & Katz, Y. β-Phenylpyruvate induces long-term neurobehavioral damage and brain necrosis in neonatal mice. Behav. Brain Res. 143, 1–5 (2003).
Cordero, M.E., Trejo, M., Colombo, M. & Aranda, V. Histological maturation of the neocortex in phenylketonuric rats. Early Hum. Dev. 8, 157–173 (1983).
Lashuel, H.A., Hartley, D., Petre, B.M., Walz, T. & Lansbury, P.T. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418, 291 (2002).
Bucciantini, M. et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416, 507–511 (2002).
Solomon, B. Clinical immunologic approaches for the treatment of Alzheimer's disease. Expert Opin. Investig. Drugs 16, 819–828 (2007).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999).
Acknowledgements
We thank R. Shaltiel-Karyo for confocal microscopy analysis, S. Wolf for the electron diffraction analysis, L. Buzhansky for help with the NMR and HPLC analysis, J. Delarea for help with TEM and SEM experiments, Z. Barkay for help with the SEM and ESEM analysis, S.-C. Jung (Ewha Womans University, Korea) for BTBR-Pahenu2 mouse plasma and tissue samples, C. Troakes (London Neurodegenerative Diseases Brain Bank, King's College London and part of BrainNet Europe) and T. Arzberger (Centre for Neuropathology and Prion Research, München) for brain tissue samples, I. Benhar and members of the Gazit laboratory for helpful discussions. L.A.-A. gratefully acknowledges the support of the Colton Foundation. This work was partly supported by the Israel Science Foundation–Legacy Heritage Biomedical Science Partnership grant 862/09 and the Alzheimer's Association grant NIRG-11-205535 (to D.F.). The work in the A.C. group was supported by the Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
L.A.-A., O.C. and E.G. conceived and designed the experiments. L.A.-A. and L.V. planned and performed the experiments. D.T., L.V. and L.A.-A. designed and performed the mouse and human histology experiments. D.F. designed and coordinated the mice and human histology experiments. A.M. designed and performed the molecular dynamics simulations. A.C. designed and coordinated the molecular dynamics simulations. L.A.-A. and E.G. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 1842 kb)
Rights and permissions
About this article
Cite this article
Adler-Abramovich, L., Vaks, L., Carny, O. et al. Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria. Nat Chem Biol 8, 701–706 (2012). https://doi.org/10.1038/nchembio.1002
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1002
This article is cited by
-
Investigation of encapsulated water wire within self-assembled hydrophilic nanochannels, in a modified γ4-amino acid crystals: Tracking thermally induced changes of intermolecular interactions within a crystalline hydrate
Amino Acids (2024)
-
A review on the self-assembly of phenylalanine as the hallmark of the neurological disorder phenylketonuria (PKU): origin to therapeutic strategy
Proceedings of the Indian National Science Academy (2023)
-
Hemostatic effects of FmocF-ADP hydrogel consisted of Fmoc-Phenylalanine and ADP
Amino Acids (2023)
-
Controlling supramolecular filament chirality of hydrogel by co-assembly of enantiomeric aromatic peptides
Journal of Nanobiotechnology (2022)
-
Single amino acid bionanozyme for environmental remediation
Nature Communications (2022)