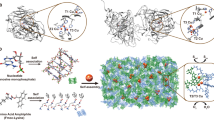
Abstract
In enzymes, the electronic and steric environments of active centres, and therefore their activity in biological processes, are controlled by the surrounding amino acids. In a similar manner, organic ligands have been used for the ‘passivation’ of metal clusters, that is, inhibition of their aggregation and control of their environment. However, the ability of enzymes to maintain large degrees of accessibility has remained difficult to mimic in synthetic systems in which little room, if any, is typically left to bind to other species. Here, using calix[4]arene macrocycles bearing phosphines as crude mimics of the rigid backbones of proteins, we demonstrate the synthesis of gold clusters and the control of their accessibility through an interplay between the sizes of the calixarene ligands and metal cores. For 0.9-nm cores, 25% of all the gold atoms within the cluster bind to the chemisorption probe 2-naphthalenethiol. This accessibility dramatically decreases with 1.1-nm and 4-nm gold cores.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Beinert, H. Iron–sulfur proteins: ancient structures, still full of surprises. J. Biol. Inorg. Chem. 5, 2–15 (2000).
Robbins, A. H. & Stout, C. D. Structure of activated aconitase: formation of the [4Fe-4S] cluster. Proc. Natl Acad. Sci. USA 86, 3639–3643 (1989).
Quintanar, L. et al. Spectroscopic and electronic structure studies of the trinuclear Cu cluster active site of the multicopper oxidase laccase: nature of its coordination unsaturation. J. Am. Chem. Soc. 127, 13832–13845 (2005).
Banavar, J. R. & Maritan, A. Physics of proteins. Annu. Rev. Biophys. Biomol. Struct. 36, 261–280 (2007).
Xu, Z. et al. Size-dependent catalytic activity of supported metal clusters. Nature 372, 346–348 (1994).
Corma, A. & Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 37, 2096–2126 (2008).
Hashmi, A. S. K. & Hutchings, G. J. Gold catalysis. Angew. Chem. Int. Ed. 45, 7896–7936 (2006).
Choundhary, T. V. & Goodman, D. W. Oxidation catalysis by supported gold nano-clusters. Top. Catal. 21, 25–34 (2002).
Corma, A. et al. Gold nanoparticles in organic capsules: a supramolecular assembly of gold nanoparticles and cucurbituril. Eur. J. Chem. 13, 6359–6364 (2007).
Nowicki, A. et al. Supramolecular shuttle and protective agent: a multiple role of methylated cyclodextrins in the chemoselective hydrogenation of benzene derivatives with ruthenium nanoparticles. Chem. Commun. 3, 296–298 (2006).
Denicourt-Nowicki, A., Ponchel, A., Monflier, E. & Roucoux, A. Methylated cyclodextrins: an efficient protective agent in water for zerovalent ruthenium nanoparticles and a supramolecular shuttle in alkene and arene hydrogenation reactions. Dalton Trans. 48, 5714–5719 (2007).
Denicourt-Nowicki, A., Roucoux, A. & Wyrwalski, F. Carbon-supported ruthenium nanoparticles stabilized by methylated cyclodextrins: a new family of heterogeneous catalysts for the gas-phase hydrogenation of arenes. Eur. J. Chem. 14, 8090–8093 (2008).
Gopidas, K. R., Whitesell, J. K. & Fox, M. A. Nanoparticle-cored dendrimers: synthesis and characterization. J. Am. Chem. Soc. 125, 6491–6502 (2003).
Crooks, R. M., Zhao, M., Sun, L., Chechik, V. & Yeung, L. K. Dendrimer-encapsulated metal nanoparticles: synthesis, characterization and applications to catalysis. Acc. Chem. Res. 34, 181–190 (2001).
Toshima, N. Nanoscopic structure and function of polymer-protected metal clusters. Macromol. Symp. 156, 45–52 (2000).
Baiker, A. Progress in asymmetric heterogeneous catalysis: design of novel chirally modified platinum metal catalysts. J. Mol. Cat. A 115, 473–493 (1997).
Ha, J.-M., Solovyov, A. & Katz, A. Postsynthetic modification of gold nanoparticles with calix[4]arene enantiomers: origin of chiral surface plasmon resonance. Langmuir 25, 153–158 (2009).
Ha, J.-M., Solovyov, A. & Katz, A. Synthesis and characterization of accessible metal surfaces in calixarene-bound gold nanoparticles. Langmuir 25, 10548–10553 (2009).
Xu, W., Puddephatt, R. J., Manojlovic-Muir, L., Muir, K. W. & Frampton, C. S. J. Calixarenes: structure of an acetonitrile inclusion complex and some transition metal rimmed derivatives. J. Incl. Phenom. 19, 277–290 (1994).
Dieleman, C. B., Matt, D. & Harriman, A. Coordination chemistry of calix-phosphanes: cooperativity in the assembly of a tetragold calixarene complex. Eur. J. Inorg. Chem. 831–834 (2000), and references therein.
Ungaro, R., Pochini, A., Andreetti, G. D. & Domiano, P. Molecular inclusion in functionalized macrocycles: the crystal and molecular-structure of para-tert-butylcalix[4]arene anisole(2–1) complex—a new type of cage compound. J. Chem. Soc. Perkin Trans. II, 197–201 (1985).
Bartlett, P. A., Bauer, B. & Singer, S. J. Synthesis of water-soluble undecagold cluster compounds of potential importance in electron microscopies and other studies of biological systems. J. Am. Chem. Soc. 100, 5085–5089 (1978).
Albano, V. G., Bellon, P. L., Manassero, M. & Sansoni, M. Intermetallic pattern in metal–atom clusters—structural studies on Au11X3(PR3)7 species. Chem. Commun. 1210–1211 (1970).
Vollenbroek, F. A., Bour, J. J. & van der Velden, J. W. A. Gold–phopshine cluster compounds—the reactions of [A9L8]3+ (L=PPh3) with L, SCN− and Cl− TO [Au8L8]2+, [Au11L8(SCN)2]+ and [Au11L8Cl2]+. Rec. Trav. Chim. Pays-Bas 99, 137–141 (1980).
Smith, J. M. M., Bour, J. J., Vollenbroek, F. A. & Beurskens, P. T. Preparation and X-ray structure determination of [pentakis(1,3-bis(diphenylphosphino)propane)] undecagold tris(thiocyante), [Au11(PPH2C3H6PPH2)5](SCN)3 . J. Cryst. Spec. Rec. 13, 355–363 (1983).
Safer, D., Lizann, B. & Leigh, J. S. Undecagold clusters for site-specific labeling of biological macromolecules: simplified preparation and model applications. J. Inorg. Biochem. 26, 77–91 (1986).
Yang, Y. & Chen, S. Surface manipulation of the electronic energy of subnanometer-sized gold clusters: an electrochemical and spectroscopic investigation. Nano Lett. 3, 75–79 (2003).
Nunokawa, K. et al. Synthesis and characterization of the Au11 cluster with sterically demanding phosphine ligands by single crystal X-ray diffraction and XPS spectroscopy. Bull. Chem. Soc. Jpn 76, 1601–1602 (2003).
Woehrle, G. H., Warner, M. G. & Hutchison, J. E. Ligand exchange reactions yield subnanometer, thiol-stabilized gold particles with defined optical transitions. J. Phys. Chem. B 106, 9979–9981 (2002).
Yanagimoto, Y., Negishi, Y., Fujihara, H. & Tsukuda, T. Chiroptical activity of BINAP-stabilized undecagold clusters. J. Phys. Chem. B 110, 11611–11614 (2006).
Hall, K. P. & Mingos, D. M. P. Homonuclear and heteronuclear cluster compounds of gold. Prog. Inorg. Chem. 32, 237–325 (1984), and references therein.
Woehrle, G. H. & Hutchison, J. E. Thiol-functionalized undecagold clusters by ligand exchange: synthesis, mechanism and properties. Inorg. Chem. 44, 6149–6158 (2005).
Tunik, S. P. et al. Synthesis and characterization of bis-(diphenylphosphinoethane)-substituted hexarhodium clusters: Rh6(CO)14(μ2,η2-Ph2P(CH2)2PPh2), Rh6(CO)152 (μ2,η1:η1-Ph2P(CH2)2 PPh2) and Rh6(CO)15(η1-Ph2 P(CH2)2P(O)Ph2). X-ray crystal structures of Rh6(CO)15PPh3 and Rh6(CO)14 (μ2,η2-Ph2P(CH2)2PPh2) clusters. J. Organomet. Chem. 433, 189–201 (1992).
Hostetler, M. J. et al. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: core and monolayer properties as a function of core size. Langmuir 14, 17–30 (1998).
De Silva, N., Solovyov, A. & Katz, A. Patterned metal polyhedra using calixarenes as organizational scaffolds: Ir4-based cluster assemblies. Dalton Trans. 39, 2194–2197 (2010).
Menard, L. D. et al. Sub-nanometer Au monolayer-protected clusters exhibiting molecule-like electronic behavior: quantitative high-angle annular dark-field scanning transmission electron microscopy and electrochemical characterization of clusters with precise atomic stoichiometry. J. Phys. Chem. B 110, 12874–12883 (2006).
Chusuei, C. C., Lai, X., Luo, K. & Goodman, D. W. Modeling heterogeneous catalysts: metal clusters on planar oxide supports. Top. Catal. 14, 71–83 (2001).
Mason, M. G. Electronic-structure of supported small metal-clusters. Phys. Rev. B 27, 748–762 (1983).
Menard, L. D. et al. Metal core bonding motifs of monodisperse icosahedral Au13 and larger Au monolayer-protected clusters as revealed by X-ray absorption spectroscopy and transmission electron microscopy. J. Phys. Chem. B 110, 14564–14573 (2006).
Veith, G. M., Lupini, A. R. & Dudney, N. J. Role of pH in the formation of structurally stable and catalytically active TiO2-supported gold catalysts. J. Phys. Chem. C 113, 269–280 (2009).
Cariati, F. & Naldini, L. Trianionoeptakis(triarylphosphine)undecagold cluster compounds. Inorg. Chim. Acta 5, 172–174 (1971).
Vollenbroek, F. A., Bour, J. J., Trooster, J. M. & van der Veldon, J. W. A. Reactions of gold–phosphine cluster compounds. Chem. Commun. 907–909 (1978).
Zhang, P. & Sham T. K. X-ray studies of the structure and electronic behavior of alkanethiolate-capped gold nanoparticles: the interplay of size and surface effects. Phys. Rev. Lett. 90, 245502 (2003).
Corma, A. & Garcia, H. Supported Gold Nanoparticles as Oxidation Catalysts in Nanoparticles and Catalysis 389–426 (Wiley VCH Verlag, 2008).
Jadzinsky, P. D., Calero, G., Ackerson, C. J., Bushnell, D. A. & Kornberg, R. D. Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Ångstrom resolution. Science 318, 430–433 (2007).
Ha, J.-M., Katz, A., Drapailo, A. B. & Kalchenko, V. I. Mercaptocalixarene-capped gold nanoparticles via postsynthetic modification and direct synthesis: effect of calixarene cavity–metal interactions. J. Phys. Chem. C 113, 1137–1142 (2009).
Jaguar, version 7.5, MacroModel, version 9.6, Schrodinger, New York (2008).
Zhao, Y. & Truhlar, D. G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157–167 (2008).
Zhao, Y., Schultz, N. E. & Truhlar, D. G. Exchange-correlation functional with broad accuracy for metallic and nonmetallic compounds, kinetics and noncovalent interactions. J. Chem. Phys. 123, 161103 (2005).
Acknowledgements
The authors are grateful to Chevron Corporation for financial support of this research. The authors acknowledge the technical assistance of R. Nichiporuk in the Mass Spectrometry Facility and National Institutes of Health grant no. 10RR022393-01 for the acquisition of the Q-Tof mass spectrometer. The authors also thank F.J. Hollander and A. Dipasquale for assistance with the characterization of 1a, 1b and 1c by means of single-crystal X-ray diffraction. The authors acknowledge the support of the National Center for Electron Microscopy, Lawrence Berkeley Lab, supported by the U.S. Department of Energy under contract no. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
A.S. designed and synthesized all organic ligands. N.d.S. designed and performed the experiments related to the synthesis of gold complexes, crystals for X-ray diffraction and gold clusters. J.-M.H. designed and performed steady-state fluorescence experiments, HAADF-STEM characterization and NMR experiments with labelled complexes and clusters. M.M.N. and I.O. synthesized labelled gold complexes and clusters. S.W.Y. provided XPS characterization of the gold clusters. K.A.D. contributed computational modelling of the gold complexes and clusters. N.d.S., J.-M.H., A.S. and A.K. conceived the experiments and co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4212 kb)
Supplementary information
Crystallographic data for compound 1a (CIF 20 kb)
Supplementary information
Crystallographic data for compound 1b (CIF 34 kb)
Supplementary information
Crystallographic data for compound 1c (CIF 31 kb)
Supplementary information
Crystallographic data for compound 2b (CIF 48 kb)
Rights and permissions
About this article
Cite this article
de Silva, N., Ha, JM., Solovyov, A. et al. A bioinspired approach for controlling accessibility in calix[4]arene-bound metal cluster catalysts. Nature Chem 2, 1062–1068 (2010). https://doi.org/10.1038/nchem.860
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.860
This article is cited by
-
Atomically precise copper dopants in metal clusters boost up stability, fluorescence, and photocatalytic activity
Communications Chemistry (2023)
-
Understanding ligand-protected noble metal nanoclusters at work
Nature Reviews Materials (2023)
-
Facile construction of calix[4]pyrrole-templated gold nanoparticles: computational insights and application for efficient reduction of 4-nitrophenol
Gold Bulletin (2019)