Abstract
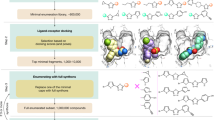
An effective and efficient means to catalyst discovery is the high-throughput screening of catalyst libraries. However, the current status of this approach suffers from a number of limitations, namely access to structurally diverse and meaningful ligand libraries and the enormous effort required for massive parallel screening of the resulting catalysts. We report an integrated solution to these drawbacks, which combines a diversity-oriented ligand synthesis, a catalyst-generation process driven by self-assembly and, finally, a combinatorial iterative library deconvolution strategy to identify the optimal catalyst. As a test case, rhodium-catalysed asymmetric hydrogenation was studied and, from a library of 120 self-assembling catalysts, highly enantioselective catalysts for the asymmetric hydrogenation of different olefinic substrates were identified within 17 experiments. Comparison of the results of the iterative library deconvolution strategy with those of the classic parallel-screening process confirmed the validity of this approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zhang, W., Chi, Y. & Zhang, Z. Developing chiral ligands for asymmetric hydrogenation. Acc. Chem. Res. 40, 1278–1290 (2007).
Tang, W. & Zhang, X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev. 103, 3029–3070 (2003).
Blaser U. & Schmidt, E. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions (Wiley, 2003).
Gennari, C. & Piarulli, U. Combinatorial libraries of chiral ligands for enantioselective catalysis. Chem. Rev. 103, 3071–3100 (2003).
Jaekel, C. & Paciello, R. High-throughput and parallel screening methods in asymmetric hydrogenation. Chem. Rev. 106, 2912–2942 (2006).
Knowles, W. S. Asymmetric hydrogenation. Acc. Chem. Res. 16, 106–112 (1983).
Mori, S., Vreven, T. & Morokuma, K. Transition states of binap-rhodium(I)-catalyzed asymmetric hydrogenation: theoretical studies on the origin of the enantioselectivity. Chem. Asian J. 1, 391–403 (2006).
Reetz, M. T., Sell, T., Meiswinkel, A. & Mehler, G. A new principle in combinatorial asymmetric transition-metal catalysis: mixtures of chiral monodentate P ligands Angew. Chem. Int. Ed. 42, 790–793 (2003).
Reetz, M. T. & Mehler, G. Mixtures of chiral and achiral monodentate ligands in asymmetric Rh-catalyzed olefin hydrogenation: reversal of enantioselectivity. Tetrahedron Lett. 44, 4593–4596 (2003).
Reetz, M. T. & Li, X. Combinatorial approach to the asymmetric hydrogenation of β-acylamino acrylates: use of mixtures of chiral monodentate P-ligands. Tetrahedron 60, 9709–9714 (2004).
Reetz, M. T. & Li, X. The influence of mixtures of monodentate achiral ligands on the regioselectivity of transition-metal-catalyzed hydroformylation. Angew. Chem. Int. Ed. 44, 2959–2962 (2005).
Pena, D. et al. Improving conversion and enantioselectivity in hydrogenation by combining different monodentate phosphoramidites; a new combinatorial approach in asymmetric catalysis. Org. Biomol. Chem. 1, 1087–1089 (2003).
Pena, D., Minnaard, A. J., de Vries, J. G. & Feringa, B. L. Highly enantioselective rhodium-catalyzed hydrogenation of β-dehydroamino acid derivatives using monodentate phosphoramidites. J. Am. Chem. Soc. 124, 14552–14553 (2002).
Duursma, A. et al. First examples of improved catalytic asymmetric C–C bond formation using the monodentate ligand combination approach. Org. Lett. 5, 3111–3113 (2003).
Monti, C., Gennari, C. & Piarulli, U. Rh-catalysed asymmetric hydrogenations with a dynamic library of chiral tropos phosphorus-ligands. Tetrahedron Lett. 45, 6859–6862 (2004).
Monti, C., Gennari, C. & Piarulli, U. Enantioselective conjugate addition of phenylboronic acid to enones catalysed by a chiral tropos/atropos rhodium complex at the coalescence temperature. Chem. Commun. 5281–5283 (2005).
Dahmen S. & Braese S. Combinatorial methods for the discovery and optimisation of homogeneous catalysts. Synthesis 10, 1431–1449 (2001).
Reetz, M. T. Combinatorial transition-metal catalysis: mixing monodentate ligands to control enantio-, diastereo-, and regioselectivity. Angew. Chem. Int. Ed. 47, 2556–2588 (2008).
Breit, B. & Seiche, W. Self-assembly of bidentate ligands for combinatorial homogeneous catalysis based on an A–T base-pair model. J. Am. Chem. Soc. 125, 6608–6609 (2003).
Breit, B. & Seiche, W. Self-assembly of bidentate ligands for combinatorial homogeneous catalysis based on an A–T base-pair model. Angew. Chem. Int. Ed. 44, 1640–1643 (2005).
Seiche, W., Schuschkowski, A. & Breit, B. Room temperature/ambient pressure regioselective hydroformylation of terminal alkenes. Adv. Synth. Catal. 347, 1488–1494 (2005).
Breit, B. & Seiche, W. Self-assembly of bidentate ligands for combinatorial homogeneous catalysis. Pure Appl. Chem. 78, 249–256 (2006).
Chevallier, F. & Breit, B. Self-assembled bidentate ligands for Ru-catalyzed anti-Markovnikov hydration of terminal alkynes. Angew. Chem. Int. Ed. 45, 1599–1602 (2006).
Birkholz, M.-N. et al. Enantioselective Pd-catalyzed allylic amination with self-assembling and non-assembling monodentate phosphine ligands. Tetrahedron: Asymmetry 18, 2055–2060 (2007).
Weis, M., Waloch, C., Seiche, W. & Breit, B. Self-assembly of bidentate ligands for combinatorial homogeneous catalysis: asymmetric rhodium-catalyzed hydrogenation. J. Am. Chem. Soc. 128, 4188–4189 (2006).
Smejkal, T. & Breit, B. Self-assembled bidentate ligands for ruthenium-catalyzed hydration of nitriles. Organometallics 26, 2461–2464 (2007).
Wieland, J., Waloch, C., Keller, M. & Breit, B. Self-assembly of bidentate ligands for combinatorial homogeneous catalysis: methanol-stable platforms analogous to the adenine thymine base pair. Angew. Chem. Int. Ed. 46, 3037–3039 (2007).
de Greef, M. & Breit, B. Self-assembled bidentate ligands for the nickel-catalyzed hydrocyanation of alkenes. Angew. Chem. Int. Ed. 48, 551–554 (2009).
Slagt, V. F., van Leeuwen, P. W. N. M. & Reek, J. N. H. Multicomponent porphyrin assemblies as functional bidentate phosphite ligands for regioselective rhodium-catalyzed hydroformylation. Angew. Chem. Int. Ed. 42, 5619–5623 (2003).
Slagt, V. F., Roeder, M., Kamer, P. C. J., van Leeuwen P. W. N. M. & Reek, J. N. H. Supraphos: a supramolecular strategy to prepare bidentate ligands. J. Am. Chem. Soc. 126, 4056–4057 (2004).
Reek, J. N. H. et al. Supraphos: a supramolecular strategy to prepare bidentate ligands. J. Organomet. Chem. 690, 4505–4516 (2005).
Jiang, X.-B. et al. Screening of a supramolecular catalyst library in the search for selective catalysts for the asymmetric hydrogenation of a difficult enamide substrate. Angew. Chem. Int. Ed. 45, 1223–1227 (2006).
Takacs, J. M., Reddy, D. S., Moteki, S. A., Wu, D. & Palencia, H. Asymmetric catalysis using self-assembled chiral bidentate P,P-ligands. J. Am. Chem. Soc. 126, 4494–4495 (2004).
Duckmanton, P. A., Blake, A. J. & Love, J. B. Palladium and rhodium ureaphosphine complexes: exploring structural and catalytic consequences of anion binding. Inorg. Chem. 44, 7708–7710 (2005).
Shi, L. et al. Engineering a polymeric chiral catalyst by using hydrogen bonding and coordination interactions Angew. Chem. Int. Ed. 45, 4108–4112 (2006).
Breuil, P.-R. R., Patureau, F. W. & Reek, J. N. H. Singly hydrogen bonded supramolecular ligands for highly selective rhodium-catalyzed hydrogenation reactions. Angew. Chem. Int. Ed. 48, 2196–2199 (2009).
Bougioukou, D. J. R., Kille, S. Taglieber, A. & Reetz M. T. Directed evolution of an enantioselective enoate-reductase: testing the utility of iterative saturation mutagenesis. Adv. Synth. Catal. 351, 3287–3305 (2009).
Polizzi K. M. et al. Simulation modeling of pooling for combinatorial protein engineering. J. Biomol. Screening 10, 865–864 (2005).
Acknowledgements
This work was funded by the German Research Foundation (International Research Training Group IRTG 1038, ‘Catalysts and Catalytic Reactions for Organic Synthesis’), the Alfried Krupp Award for young university teachers of the Krupp foundation (to B.B.) and BASF SE. We thank A. Lutterer, S. Ikkes, H. Ziegler and G. Leonhard-Lutterbeck for technical assistance.
Author information
Authors and Affiliations
Contributions
J.W. and B.B. conceived and designed the experiments. J.W. performed the experiments. J.W. and B.B. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wieland, J., Breit, B. A combinatorial approach to the identification of self-assembled ligands for rhodium-catalysed asymmetric hydrogenation. Nature Chem 2, 832–837 (2010). https://doi.org/10.1038/nchem.800
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.800
This article is cited by
-
Ligand relay catalysis for cobalt-catalyzed sequential hydrosilylation and hydrohydrazidation of terminal alkynes
Nature Communications (2022)
-
Ni-catalyzed highly enantioselective synthesis of sulfur protected P-stereogenic supramolecular phosphine
Journal of Chemical Sciences (2021)
-
Cationic Organic Catalysts or Ligands in Concert with Metal Catalysts
Topics in Current Chemistry (2019)
-
By-design enantioselective self-amplification based on non-covalent product–catalyst interactions
Nature Chemistry (2017)
-
The power of pairing
Nature Chemistry (2012)