Abstract
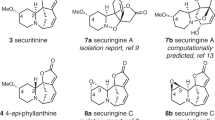
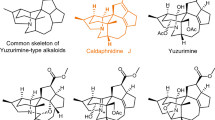
The cortistatins are a recently identified class of marine natural products characterized by an unusual steroidal skeleton, which have been found to inhibit differentially the proliferation of various mammalian cells in culture by an unknown mechanism. We describe a comprehensive route for the synthesis of cortistatins from a common precursor, which in turn is assembled from two fragments of similar structural complexity. Cortistatins A and J, and for the first time K and L, have been synthesized in parallel processes from like intermediates prepared from a single compound. With the identification of facile laboratory transformations linking intermediates in the cortistatin L synthetic series with corresponding intermediates to cortistatins A and J, we have been led to speculate that somewhat related paths might occur in nature, offering potential sequencing and chemical detail for cortistatin biosynthetic pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Aoki, S. et al. Cortistatins A, B, C and D, anti-angiogenic steroidal alkaloids, from the marine sponge Corticium simplex. J. Am. Chem. Soc. 128, 3148–3149 (2006).
Watanabe, Y., Aoki, S., Tanabe, D., Setiawan, A. & Kobayashi, M. Cortistatins E, F, G and H, four novel steroidal alkaloids from marine sponge Corticium simplex. Tetrahedron 63, 4074–4079 (2007).
Aoki, S. et al. Cortistatins J, K, L, novel abeo-9(10-19)-androstane-type steroidal alkaloids with isoquinoline unit, from marine sponge Corticium simplex. Tetrahedron Lett. 48, 4485–4488 (2007).
Aoki, S. et al. Structure–activity relationship and biological property of cortistatins, anti-angiogenic spongean steroidal alkaloids. Bioorg. Med. Chem. 15, 6758–6762 (2007).
Shenvi, R. A., Guerrero, C. A., Shi, J., Li, C.-C. & Baran, P. S. Synthesis of (+)-cortistatin A. J. Am. Chem. Soc. 130, 7241–7243 (2008).
Nicolaou, K. C., Sun, Y. P., Peng, X. S., Polet, D. & Chen, D. Y. K. Total synthesis of (+)-cortistatin A. Angew. Chem. Int. Ed. 47, 7310–7313 (2008).
Lee, H. M., Nieto-Oberhuber, C. & Shair, M. D. Enantioselective synthesis of (+)-cortistatin A, a potent and selective inhibitor of endothelial cell proliferation. J. Am. Chem. Soc. 130, 16864–16866 (2008).
Simmons, E. M., Hardin, A. R., Guo, X. & Sarpong, R. Rapid construction of the cortistatin pentacyclic core. Angew. Chem. Int. Ed. 47, 6650–6653 (2008).
Yamashita, S., Iso, K. & Hirama, M. A concise synthesis of the pentacyclic framework of cortistatins. Org. Lett. 10, 3413–3415 (2008).
Craft, D. T. & Gung, B. W. The first transannular [4 + 3] cycloaddition reaction: synthesis of the ABCD ring structure of cortistatins. Tetrahedron Lett. 49, 5931–5934 (2008).
Dai, M. J. & Danishefsky, S. J. A concise synthesis of the cortistatin core. Tetrahedron Lett. 49, 6610–6612 (2008).
Dai, M. J., Wang, Z. & Danishefsky, S. J. A novel α,β–unsaturated nitrone-aryne [3 + 2] cycloaddition and its application in the synthesis of the cortistatin core. Tetrahedron Lett. 49, 6613–6616 (2008).
Kotoku, N., Sumii, Y., Hayashi, T. & Kobayashi, M. Synthesis of CD-ring structure of cortistatin A, an anti-angiogenic steroidal alkaloid from marine sponge. Tetrahedron Lett. 49, 7078–7081 (2008).
Kurti, L., Czako, B. & Corey, E. J. A short, scalable synthesis of the carbocyclic core of the anti-angiogenic cortistatins from (+)-estrone by B-ring expansion. Org. Lett. 10, 5247–5250 (2008).
Dai, M. J. & Danishefsky, S. J. An oxidative dearomatization cyclization model for cortistatin A. Heterocycles 77, 157–161 (2009).
Liu, L. Z. et al. A model study for the concise construction of the oxapentacyclic core of cortistatins through intramolecular Diels–Alder and oxidative dearomatization–cyclization reactions. Chem. Commun. 662–664 (2009).
Magnus, P. & Littich, R. Intramolecular cyclopropene-furan [2 + 4] cycloaddition followed by a cyclopropylcarbinyl rearrangement to synthesize the BCD rings of cortistatin A. Org. Lett. 11, 3938–3941 (2009).
Frie, J. L., Jeffrey, C. S. & Sorensen, E. J. A hypervalent iodine-induced double annulation enables a concise synthesis of the pentacyclic core structure of the cortistatins. Org. Lett. 11, 5394–5397 (2009).
Yamashita, S., Kitajima, K., Iso, K. & Hirama, M. Efficient and stereoselective installation of isoquinoline: formal total synthesis of cortistatin A. Tetrahedron Lett. 50, 3277–3279 (2009).
Simmons, E. M., Hardin-Narayan, A. R., Guo, X. & Sarpong, R. Formal total synthesis of (±)-cortistatin A. Tetrahedron 66, 4696–4700 (2010).
Nicolaou, K. C. et al. Total synthesis and biological evaluation of cortistatins A and J and analogues thereof. J. Am. Chem. Soc. 131, 10587–10597 (2009).
Shi, J. et al. Stereodivergent synthesis of 17-α and 17-β-aryl steroids: application and biological evaluation of D-ring cortistatin analogues. Angew. Chem. Int. Ed. 48, 4328–4331 (2009).
Czako, B., Kurti, L., Mammoto, A., Ingber, D. E. & Corey, E. J. Discovery of potent and practical antiangiogenic agents inspired by cortistatin A. J. Am. Chem. Soc. 131, 9014–9019 (2009).
Cee, V. J., Chen, D. Y. K., Lee, M. R. & Nicolaou, K. C. Cortistatin A is a high-affinity ligand of protein kinases ROCK, CDK8 and CDK11. Angew. Chem. Int. Ed. 48, 8952–8957 (2009).
Berk, S. C., Knochel, P. & Yeh, M. C. P. General approach to highly functionalized benzylic organometallics of zinc and copper. J. Org. Chem. 53, 5789–5791 (1988).
Pearson, D. E., Cowan, D. & Beckler, J. D. A study of the entrainment method for making Grignard reagents. J. Org. Chem. 24, 504–509 (1959).
Isaacs, R. C. A., Digrandi, M. J. & Danishefsky, S. J. Synthesis of an enantiomerically pure intermediate containing the CD substructure of taxol. J. Org. Chem. 58, 3938–3941 (1993).
Hajos, Z. G. & Parrish, D. R. (+)-(7aS)-7a-methyl-2,3,7,7a-tetrahydro-1H-indene-1,5-6H-dione. In Organic Syntheses, Vol. 63, 26–31 (Wiley & Sons, 1985).
Evans, D. A., Hurst, K. M. & Takacs, J. M. New silicon-phosphorus reagents in organic synthesis: carbonyl and conjugate addition reactions of silicon phosphite esters and related systems. J. Am. Chem. Soc. 100, 3467–3477 (1978).
Kozikowski, A. P. & Jung, S. H. Phosphoniosilylation: an efficient and practical method for the β-functionalization of enones. J. Org. Chem. 51, 3400–3402 (1986).
Mi, Y., Schreiber, J. V. & Corey, E. J. Total synthesis of (+)-α-onocerin in four steps via four-component coupling and tetracyclization steps. J. Am. Chem. Soc. 124, 11290–11291 (2002).
Walker, S. D., Barder, T. E., Martinelli, J. R. & Buchwald, S. L. A rationally designed universal catalyst for Suzuki–Miyaura coupling processes. Angew. Chem. Int. Ed. 43, 1871–1876 (2004).
Scholl, M., Ding, S., Lee, C. W. & Grubbs, R. H. Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands. Org. Lett. 1, 953–956 (1999).
Murray, R. W. & Singh, M. Synthesis of epoxides using dimethyldioxirane: trans-stilbene oxide. In Organic Syntheses, Vol. 74, 91–100 (Wiley & Sons, 1997).
Thummel, R. P. & Rickborn, B. Base-induced rearrangement of epoxides. V. Phenyl-substituted epoxides. J. Org. Chem. 37, 3919–3923 (1972).
Kita, Y., Tohma, H., Kikuchi, K., Inagaki, M. & Yakura, T. Hypervalent iodine oxidation of N-acyltyramines: synthesis of quinol ethers, spirohexadienones and hexahydroindol-6-ones. J. Org. Chem. 56, 435–438 (1991).
Kogure, T. & Ojima, I. Reduction of carbonyl compounds via hydrosilylation. 4. Highly regioselective reductions of α,β-unsaturated carbonyl compounds. Organometallics 1, 1390–1399 (1982).
Corey, E. J. & Helal, C. J. Reduction of carbonyl compounds with chiral oxazaborolidine catalysts: a new paradigm for enantioselective catalysis and a powerful new synthetic method. Angew. Chem. Int. Ed. 37, 1986–2012 (1998).
San Filippo, J., Chern, C. I. & Valentine, J. S. Reaction of superoxide with alkyl halides and tosylates. J. Org. Chem. 40, 1678–1680 (1975).
Corey, E. J., Nicolaou, K. C., Shibasaki, M., Machida, Y. & Shiner, C. S. Superoxide ion as a synthetically useful oxygen nucleophile . Tetrahedron Lett. 37, 3183–3186 (1975).
Ishihara, K., Kubota, M., Kurihara, H. & Yamamoto, H. Scandium trifluoromethanesulfonate as an extremely active acylation catalyst. J. Am. Chem. Soc. 117, 4413–4414 (1995).
Hutchins, R. O., Learn, K. & Fulton, R. P. Reductive displacement of allylic acetates by hydride transfer via catalytic activation by palladium(0) complexes. Tetrahedron Lett. 21, 27–30 (1980).
Couturier, M., Tucker, J. L., Andresen, B. M., Dube, P. & Negri, J. T. Palladium and Raney nickel catalyzed methanolic cleavage of stable borane–amine complexes. Org. Lett. 3, 465–467 (2001).
Moon, S. S., Stuhmiller, L. M. & McMorris, T. C. Synthesis of oogoniol. J. Org. Chem. 54, 26–28 (1989).
Foy, N. et al. Synthesis, receptor binding, molecular modeling and proliferative assays of a series of 17α-arylestradiols. Chembiochem. 4, 494–503 (2003).
Jang, D. O., Kim, J. G., Cho, D. H. & Chung, C. M. Radical deoxygenation of alcohols via their trifluoroacetate derivatives with diphenylsilane. Tetrahedron Lett. 42, 1073–1075 (2001).
Kim, J. G., Cho, D. H. & Jang, D. O. Radical deoxygenation of tertiary alcohols via trifluoroacetates. Tetrahedron Lett. 45, 3031–3033 (2004).
D' Auria, M. V., Minale, L. & Riccio, R. Polyoxygenated steroids of marine origin. Chem. Rev. 93, 1839–1895 (1993).
Sarma, N. S., Krishna, M. S. R. & Rao, S. R. Sterol ring system oxidation pattern in marine sponges. Mar. Drugs 3, 84–111 (2005).
Acknowledgements
Financial support from the National Institutes of Health (Stimulus grant no. CA047148-22S1) is gratefully acknowledged. A.N.F. acknowledges a scholarship from Eli Lilly and Company. We gratefully acknowledge G. Zou for measuring the GI50 values of cortistatins A, J, K and L and A.W.G. Burgett and M.D. Shair for their assistance with these measurements as well as a gift of the HUVEC. We acknowledge helpful discussions with C. T. Walsh and E. Balskus.
Author information
Authors and Affiliations
Contributions
A.N.F., C.S. and A.G.M conceived the synthetic route. A.N.F and C.S. conducted all experimental work and analysed the results. A.N.F., C.S. and A.G.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4677 kb)
Rights and permissions
About this article
Cite this article
Flyer, A., Si, C. & Myers, A. Synthesis of cortistatins A, J, K and L. Nature Chem 2, 886–892 (2010). https://doi.org/10.1038/nchem.794
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.794
This article is cited by
-
Towards a general diastereoselective route to oxabicyclo[3.2.1]octanes via a gold-catalysed cascade reaction
Nature Communications (2015)
-
Mediator kinase inhibition further activates super-enhancer-associated genes in AML
Nature (2015)
-
Clearing the way to cortistatins
Nature Chemistry (2010)