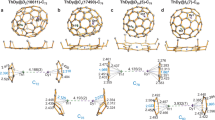
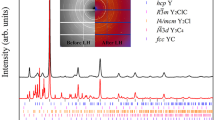
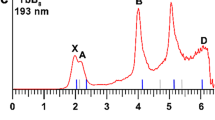
Abstract
Although metal–metal bonding is important in the chemistry of both solid-state intermetallic compounds and molecular species, the study of this bonding is limited by the compounds available and it is rarely possible to identify connections between these two areas. In this study, molecular intermetalloids [Ln(ReCp2)3] (Ln = Sm, Lu and La) have been synthesized that contain lanthanoid metals bound only to transition metals. Although they are highly reactive species, such lanthanoid-core transition-metal-shell compounds can be stable in solution. They mimic the bonding situation of intermetallic compounds, as revealed by a direct comparison of molecular and solid state lanthanoid–transition metal bonding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pauling, L. The Nature of the Chemical Bond 3rd edn (VCH, 1973).
Lewis, G. N. The atom and the molecule. J. Am. Chem. Soc. 38, 762–785 (1916).
Wagner, F. R., Noor, A. & Kempe, R. Ultrashort metal–metal distances and extreme bond orders. Nature Chem. 1, 529–536 (2009).
Nguyen, T. et al. Synthesis of a stable compound with fivefold bonding between two chromium(I) centers. Science 310, 844–847 (2005).
Resa, I., Carmona, E., Gutierrez-Puebla, E. & Monge, A. Decamethyldizincocene, a stable compound of Zn(I) with a Zn–Zn Bond. Science 305, 1136–1138 (2004).
Green, S. P., Jones, C. & Stasch, A. Stable magnesium(I) compounds with Mg–Mg bonds. Science 318, 1754–1757 (2007).
Hill, M. S., Hitchcock, P. B. & Pongtavornpinyo, R. A linear homocatenated compound containing six indium centers. Science 311, 1904–1907 (2006).
Casey, C. P., Jordan, R. F. & Rheingold, A. L. (C5H5)2Zr[Ru(CO)2C5H5]2. A metal–metal bonded zirconiumdiruthenium complex. Organometallics 3, 504–506 (1984).
Gade, L. H. Highly polar metal-metal bonds in ‘early-late’ heterodimetallic complexes Angew. Chem. Int. Ed. 39, 2658–2678 (2000).
Braunstein, P., Oro, L. A. & Raithby, P. R. Metal Clusters in Chemistry 1–3 (Wiley-VCH, 1999).
Cadenbach, T. et al. Twelve one-electron ligands coordinating one metal center: structure and bonding of [Mo(ZnCH3)9(ZnCp*)3]. Angew. Chem. Int. Ed. 47, 9150–9154 (2008).
Cadenbach, T., Gemel, Ch. & Fischer, R. A. Molecular cut-outs of Mo/Zn Hume–Rothery phases: synthesis and structure of [{Mo(CO)4}4(Zn)6(ZnCp*)4]. Angew. Chem. Int. Ed. 47, 9146–9149 (2008).
Cadenbach, T. et al. Substituent-free gallium by hydrogenolysis of coordinated GaCp*: synthesis and structure of highly fluxional [Ru2(Ga)(GaCp*)7(H)3] Angew. Chem. Int. Ed. 48, 3872–3876 (2009).
Cadenbach, T. et al. Molecular alloys, linking organometallics with intermetallic hume-rothery phases: the highly coordinated transition metal compounds [M(ZnR)n] (n ≥ 8) containing organo-zinc ligands. J. Am. Chem. Soc. 131, 16063–16077 (2009).
Gade L. H. Mercury, structural element and source of localized reactivity in metal clusters. Angew. Chem. Int. Ed. 32, 21–40 (1993).
Beletskaya, I. P. et al. Bimetallic lanthanide complexes with lanthanide-transition metal bonds. Molecular structure of (C4H8O)(C5H5)2LuRu(CO)2(C5H5). The use of 139La NMR spectroscopy. J. Am. Chem. Soc. 115, 3156–3166 (1993).
Kempe, R., Noss, H. & Irrgang, T. Towards f and d electron interactions in amido metal complexes. J. Organomet. Chem. 647, 12–20, (2002).
Liddle, S. T. Non-traditional ligands in f-block chemistry. Proc. R. Soc. A 465, 1673–1700 (2009).
Liddle, S. T. & Mills, D. P. Metal–metal bonds in f-element chemistry. Dalton Trans. 5592–5605 (2009).
Butovskii, M. V., Tok, O. L., Wagner, F. R. & Kempe, R. Bismetallocenes: lanthanoid transition metal bonds through alkane elimination. Angew. Chem. Int. Ed. 47, 6469–6472 (2008).
Arnold, P. L., McMaster, J. & Liddle, S. T. An unsupported transition metal–lanthanide bond; synthesis and crystal structure of an Nd–Fe amido N-heterocyclic carbene complex. Chem. Commun. 818–820 (2009).
Fidler, J., Suess, D. & Schrefl, T. in Handbook of Magnetism and Magnetic Materials, Vol. 4: Novel Materials 1945–1968 (Eds Kronmüller, H. & Parkin, S.) (John Wiley & Sons, 2007).
Goll, D. & Kronmüller, H. High-performance permanent magnets. Naturwissenschaften 87, 423–438 (2000).
David, E. An overview of advanced materials for hydrogen storage. J. Mat. Proc. Techn., 162–163, 169–177 (2005).
Schumann, H., Freckmann, D. M. M. & Dechert, S. Organometallic compounds of the lanthanides 157; the molecular structure of tris(trimethylsilylmethyl)samarium, -erbium, -ytterbium, and –lutetium. Z. Anorg. Allg. Chem. 628, 2422–2426 (2002).
Hitchcock, P. B., Lappert, M. F., Smith, R. G., Bartlett, R. A. & Power, P. P. Synthesis and structural characterisation of the first neutral homoleptic lanthanide metal(III) alkyls: [LnR3][Ln=La or Sm, R=CH(SiMe3)2]. J. Chem. Soc., Chem. Commun. 1007–1009 (1988).
Emsley, J. The Elements 2nd edn (Clarendon Press, 1991).
Savitskii, E. M. & Khamidov, O. K. Crystal structure of compounds of rhenium with praseodymium. Inorg. Mater. (Engl. Transl.) 1, 1479–1480 (1965); Izv. Akad. Nauk SSSR, Neorg. Mater. (Russian) 1, 1621–1622 (1965).
Kuz'ma, Yu. B. & Svarichevskaya, S. I. Crystal structure of the compound Y2ReB6 and its analogs. Sov. Phys. Crystallogr. (Engl. Transl.) 17, 569–571 (1972); Kristallografiya (Russian) 17, 658–661 (1972).
Kohout, M. Bonding indicators from electron pair density functionals. Faraday Discuss. 135, 43–54 (2007).
Wagner, F. R., Bezugly, V., Kohout, M. & Grin, Y. Charge decomposition analysis of the electron localizability indicator: a bridge between the orbital and direct space representation of the chemical bond. Chem. Eur. J. 13, 5724–5741 (2007).
Jansen, G. et al. Unsupported Ti-Co and Zr-Co Bonds in heterobimetallic complexes: a theoretical description of metal-metal bond polarity J. Am. Chem. Soc. 120, 7239–7251 (1998).
Bader, R. F. W. Atoms in Molecules: A Quantum Theory (Oxford Univ. Press, 1994).
Raub, S. & Jansen, G. A quantitative measure of bond polarity from the electron localization function and the theory of atoms in molecules. Theor. Chem. Acc. 106, 223–232 (2001).
Bickelhaupt, F. M. & Baerends, E. J. Kohn-Sham density functional theory: predicting and understanding chemistry. Rev. Comput. Chem. 15, 1–86 (2000).
Gardner, B. M., McMaster, J., Lewis, W. & Liddle, S. T. Synthesis and structure of [{N(CH2CH2NSiMe3)3}UReCp2]: a heterobimetallic complex with an unsuppported uranium-rhenium bond. Chem. Commun. 2852–2853 (2009).
Kohout, M., Wagner, F. R. & Grin, Yu. Electron localization function for transition-metal compounds. Theor. Chem. Acc. 108, 150–156 (2002).
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft, SPP 1166 ‘Lanthanoid-spezifische Funktionalitäten in Molekül und Material’ for financial support. We acknowledge U. Schwarz and W. Schnelle for IR and magnetization measurements, respectively.
Author information
Authors and Affiliations
Contributions
M.V.B. carried out the synthesis experiments and analysed the NMR spectroscopic data. C.D. performed the X-ray single crystal structure analyses. V.B. carried out the computations of the organometallic molecule. F.R.W. and Y.G. performed the analysis of structural analogies and chemical bonding. R.K. originated the central idea and wrote the manuscript with contributions from all the co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1989 kb)
Supplementary information
Crystallographic data for compound 1 (CIF 23 kb)
Supplementary information
Crystallographic data for compound 2 (CIF 22 kb)
Supplementary information
Crystallographic data for compound 3 (CIF 24 kb)
Supplementary information
Crystallographic data for compound 4 (CIF 32 kb)
Rights and permissions
About this article
Cite this article
Butovskii, M., Döring, C., Bezugly, V. et al. Molecules containing rare-earth atoms solely bonded by transition metals. Nature Chem 2, 741–744 (2010). https://doi.org/10.1038/nchem.718
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.718
This article is cited by
-
An intermetallic molecular nanomagnet with the lanthanide coordinated only by transition metals
Nature Communications (2022)
-
On-surface preparation of coordinated lanthanide-transition-metal clusters
Nature Communications (2021)
-
Transition-metal-bridged bimetallic clusters with multiple uranium–metal bonds
Nature Chemistry (2019)
-
Understanding of multimetallic cluster growth
Nature Communications (2016)