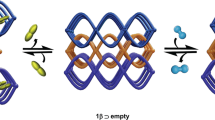
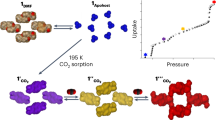
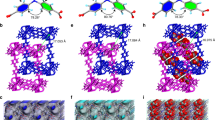
Abstract
Porous coordination polymers are materials formed from metal ions that are bridged together by organic linkers and that can combine two seemingly contradictory properties—crystallinity and flexibility. Porous coordination polymers can therefore create highly regular yet dynamic nanoporous domains that are particularly promising for sorption applications. Here, we describe the effective selective sorption of dioxygen and nitric oxide by a structurally and electronically dynamic porous coordination polymer built from zinc centres and tetracyanoquinodimethane (TCNQ) as a linker. In contrast to a variety of other gas molecules (C2H2, Ar, CO2, N2 and CO), O2 and NO are accommodated in its pores. This unprecedented preference arises from the concerted effect of the charge-transfer interaction between TCNQ and these guests, and the switchable gate opening and closing of the pores of the framework. This system provides further insight into the efficient recognition of small gas molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Batten, S. R. & Robson, R. Interpenetrating nets: ordered, periodic entanglement. Angew. Chem. Int. Ed. 37, 1460–1494 (1998).
Blake, A. J. et al. Inorganic crystal engineering using self-assembly of tailored building-blocks. Coord. Chem. Rev. 183, 117–138 (1999).
Eddaoudi, M. et al. Modular chemistry: secondary building units as a basis for the design of highly porous and robust metal–organic carboxylate frameworks. Acc. Chem. Res. 34, 319–330 (2001).
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Fletcher, A. J., Thomas, K. M. & Rosseinsky, M. J. Flexibility in metal–organic framework materials: impact on sorption properties. J. Solid State Chem. 178, 2491–2510 (2005).
Lee, J. et al. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 38, 1450–1459 (2009).
Férey, G. & Serre, C. Large breathing effects in three-dimensional porous hybrid matter: facts, analyses, rules and consequences. Chem. Soc. Rev. 38, 1380–1399 (2009).
Murray, L. J., Dincă, M. & Long, J. R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 38, 1294–1314 (2009).
Li, J. R., Kuppler, R. J. & Zhou, H. C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 38, 1477–1504 (2009).
Matsuda, R. et al. Highly controlled acetylene accommodation in a metal–organic microporous material. Nature 436, 238–241 (2005).
Mulfort, K. L. & Hupp, J. T. Chemical reduction of metal–organic framework materials as a method to enhance gas uptake and binding. J. Am. Chem. Soc. 129, 9604–9605 (2007).
Zhang, J. P. & Kitagawa, S. Supramolecular isomerism, framework flexibility, unsaturated metal center and porous property of Ag(i)/Cu(i) 3,3′,5,5′-tetrametyl-4,4′-bipyrazolate. J. Am. Chem. Soc. 130, 907–917 (2008).
Tanabe, K. K., Wang, Z. Q. & Cohen, S. M. Systematic functionalization of a metal–organic framework via a postsynthetic modification approach. J. Am. Chem. Soc. 130, 8508–8517 (2008).
Li, Q. W. et al. Docking in metal–organic frameworks. Science 325, 855–859 (2009).
Horike, S., Shimomura, S. & Kitagawa, S. Soft porous crystals. Nature Chem. 1, 695–704 (2009).
Kitaura, R., Seki, K., Akiyama, G. & Kitagawa, S. Porous coordination-polymer crystals with gated channels specific for supercritical gases. Angew. Chem. Int. Ed. 42, 428–431 (2003).
Tanaka, D. et al. Kinetic gate-opening process in a flexible porous coordination polymer. Angew. Chem. Int. Ed. 47, 3914–3918 (2008).
Hamon, L. et al. Co-adsorption and separation of CO2−CH4 mixtures in the highly flexible MIL-53(Cr) MOF. J. Am. Chem. Soc. 131, 17490–17499 (2009).
Kim, H. et al. Temperature-triggered gate opening for gas adsorption in microporous manganese formate. Chem. Commun. 4697–4699 (2008).
Koros, W. J. & Fleming, G. K. Membrane-based gas separation. J. Membr. Sci. 83, 1–80 (1993).
Stern, S. A. Polymers for gas separations—the next decade. J. Membr. Sci. 94, 1–65 (1994).
Reid, C. R. & Thomas, K. M. Adsorption of gases on a carbon molecular sieve used for air separation: linear adsorptives as probes for kinetic selectivity. Langmuir 15, 3206–3218 (1999).
Bae, Y. S. & Lee, C. H. Sorption kinetics of eight gases on a carbon molecular sieve at elevated pressure. Carbon 43, 95–107 (2005).
Yoon, J. W. et al. Gas-sorption selectivity of CUK-1: a porous coordination solid made of cobalt(II) and pyridine-2,4-dicarboxylic acid. Adv. Mater. 19, 1830–1834 (2007).
Bastin, L. et al. A microporous metal-organic framework for separation of CO2/N2 and CO2/CH4 by fixed-bed adsorption. J. Phys. Chem. C 112, 1575–1581 (2008).
Cheon, Y. E., Park, J. & Suh, M. P. Selective gas adsorption in a magnesium-based metal–organic framework. Chem. Commun. 5436–5438 (2009).
Xiao, B. et al. Chemically blockable transformation and ultraselective low-pressure gas adsorption in a non-porous metal organic framework. Nature Chem. 1, 289–294 (2009).
Vaidhyanathan, R., Iremonger, S. S., Dawson, K. W. & Shimizu, G. K. H. An amine-functionalized metal organic framework for preferential CO2 adsorption at low pressures. Chem. Commun. 5230–5232 (2009).
Golden, T. C. & Sircar, S. Gas-adsorption on silicalite. J. Colloid Interface Sci. 162, 182–188 (1994).
Shimomura, S., Horike, S., Matsuda, R. & Kitagawa, S. Guest-specific function of a flexible undulating channel in a 7,7,8,8-tetracyano-p-quinodimethane dimer-based porous coordination polymer. J. Am. Chem. Soc. 129, 10990–10991 (2007).
Meyer, E. A., Castellano, R. K. & Diederich, F. Interactions with aromatic rings in chemical and biological recognition. Angew. Chem. Int. Ed. 42, 1210–1250 (2003).
Zhao, H. et al. Spectroscopic, thermal and magnetic properties of metal/TCNQ network polymers with extensive supramolecular interactions between layers. Chem. Mater. 11, 736–746 (1999).
Ballester, L., Gutiérrez, A., Perpinán, M. F., Azcondo, M. T. & Sánchez, A. E. Interactions of TCNQ in iron and nickel coordination compounds. Synth. Met. 120, 965–966 (2001).
Kaim, W. & Moscherosch, M. The coordination chemistry of TCNE, TCNQ and related polynitrile pi-acceptors. Coord. Chem. Rev. 129, 157–193 (1994).
Khatkale, M. S. & Devlin, J. P. Vibrational and electronic-spectra of the monoanion, dianion and trianion salts of TCNQ. J. Chem. Phys. 70, 1851–1859 (1979).
Ballester, L., Gutiérrez, A., Perpinán, M. F. & Azcondo, M. T. Supramolecular architectures in low dimensional TCNQ compounds containing nickel and copper polyamine fragments. Coord. Chem. Rev. 192, 447–470 (1999).
Shamir, J., Binenboy, J. & Claassen, H. H. Vibrational frequency of O2+ cation. J. Am. Chem. Soc. 90, 6223–6224 (1968).
Smardzew, R. R. & Andrews, L. Raman spectra of products of Na and K atom argon matrix reactions with O2 molecules. J. Chem. Phys. 57, 1327–1333 (1972).
Bier, K. D. & Jodl, H. J. Influence of temperature on elementary excitations in solid oxygen by Raman studies. J. Chem. Phys. 81, 1192–1197 (1984).
Vogel, K. M., Kozlowski, P. M., Zgierski, M. Z. & Spiro, T. G. Determinants of the FeXO (X = C, N, O) vibrational frequencies in heme adducts from experiment and density functional theory. J. Am. Chem. Soc. 121, 9915–9921 (1999).
Imai, J., Souma, M., Ozeki, S., Suzuki, T. & Kaneko, K. Reaction of dimerized NOx (X = 1 or 2) with SO2 in a restricted slit-shaped micropore space. J. Phys. Chem. 95, 9955–9960 (1991).
Honda, H. et al. Choking effect of single-wall carbon nanotubes on solvent adsorption in radial breathing mode. J. Phys. Chem. C 111, 3220–3223 (2007).
Creighton, J. A. & Lippincott, E. R. Vibrational frequency and dissociation energy of superoxide ion. J. Chem. Phys. 40, 1779–1780 (1964).
Gao, Z. X., Kim, H. S., Sun, Q., Stair, P. C. & Sachtler, W. M. H. UV-Raman characterization of iron peroxo adsorbates on Fe/MFI catalyst with high activity for NOx reduction. J. Phys. Chem. B 105, 6186–6190 (2001).
Fujita, S. et al. Oxidative destruction of hydrocarbons on a new zeolite-like crystal of Ca12Al10Si4O35 including O2− and O2−2 radicals. Chem. Mater. 15, 255–263 (2003).
Acknowledgements
The authors thank S. Sakaki (Kyoto University) for his help in establishing the theoretical approach. XRPD experiments were performed at the BL02B2 in SPring-8 (proposal no. 2008B1263). This work was supported by an Exploratory Research for Advanced Technology (ERATO) project by Japan Science and Technology Agency (JST) ‘Kitagawa Integrated Pores Project’, and Riken Project in ‘Quantum Order Research Program’. Computation time was provided by the SuperComputer Laboratory, Institute for Chemical Research, Kyoto University.
Author information
Authors and Affiliations
Contributions
S.S. and S.K. designed and conceived the experiments with support from K.Y. and Y.H. S.S., M.H. and Y.M. measured the Raman spectra. S.S., M.H., R.M., Y.K., J.K. and M.T. performed XRPD measurements and analysis. S.S., R.M. and S.K. contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1305 kb)
Supplementary information
Crystallographic information for compound 1 with tetrafluorobenzene guests (CIF 22 kb)
Supplementary information
Crystallographic information for compound 1 with anisole guests (CIF 23 kb)
Supplementary information
Crystallographic information for compound 1 with benzonitrile guests (CIF 23 kb)
Supplementary information
Crystallographic information for compound 1 with p-xylene guests guests (CIF 28 kb)
Rights and permissions
About this article
Cite this article
Shimomura, S., Higuchi, M., Matsuda, R. et al. Selective sorption of oxygen and nitric oxide by an electron-donating flexible porous coordination polymer. Nature Chem 2, 633–637 (2010). https://doi.org/10.1038/nchem.684
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.684
This article is cited by
-
Selective sorption of oxygen and nitrous oxide by an electron donor-incorporated flexible coordination network
Communications Chemistry (2023)
-
Temperature-dependent rearrangement of gas molecules in ultramicroporous materials for tunable adsorption of CO2 and C2H2
Nature Communications (2023)
-
Architectural View of Flexible Aliphatic –OH Group Coordinated Hemi-Directed Pb(II)-Salen Coordination Polymer: Synthesis, Crystal Structure, Spectroscopic Insights, Supramolecular Topographies, and DFT Perspective
Journal of Inorganic and Organometallic Polymers and Materials (2022)
-
Orthogonal-array dynamic molecular sieving of propylene/propane mixtures
Nature (2021)
-
High selective photocatalytic CO2 conversion into liquid solar fuel over a cobalt porphyrin-based metal–organic framework
Photochemical & Photobiological Sciences (2021)