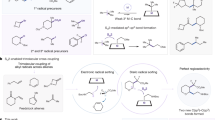
Abstract
The cleavage of ethers is commonly encountered in organometallic chemistry, although rarely studied in the context of new, emerging bimetallic reagents. Recently, it was reported that a bimetallic sodium–zinc base can deprotonate cyclic tetrahydrofuran under mild conditions without opening its heterocyclic (OC4) ring. In marked contrast to this synergic sedation, herein we show that switching to the more reactive sodium–magnesium or sodium–manganese bases promotes cleavage of at least six bonds in tetrahydrofuran, but uniquely the ring fragments are captured in separate crystalline complexes. Oxide fragments occupy guest positions in bimetallic, inverse crown ethers and C4 fragments ultimately appear in bimetallated butadiene molecules. These results demonstrate the special synergic reactivity that can be executed by bimetallic reagents, which include the ability to capture and control, and thereby study, reactive fragments from sensitive substrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Maercker, A. Ether cleavage with organo-alkali-metal compounds and alkali metals. Angew. Chem. Int. Ed. Engl. 26, 972–989 (1987).
Schlosser, M. Organometallics in Synthesis—A Manual 2nd edn (Wiley, 2002).
Clayden, J. Organolithiums: Selectivity for Synthesis (Elsevier, 2002).
Rappaport, Z. & Marek, I. Organomagnesium Compounds 2nd edn (Wiley, 2008).
Schöllkopf, U. Recent results in carbanion chemistry. Angew. Chem. Int. Ed. Engl. 9, 763–773 (1970).
Gilman, H. & Gaj, B. J. Preparation and stability of some organolithium compounds in tetrahydrofuran. J. Org. Chem. 22, 1165–1168 (1957).
Bates, R. B., Kroposki, L. M. & Potter, D. E. Cycloreversions of anions from tetrahydrofurans. A convenient synthesis of lithium enolates of aldehydes. J. Org. Chem. 37, 560–562 (1972).
Clayden, J. & Yasin, S. A. Pathways for decomposition of THF by organolithiums: the role of HMPA. New J. Chem. 26, 191–192 (2002).
Fleming, I., Mack, S. R. & Clark, B. P. α-Amino carbene or carbenoid formation in the reaction of a tertiary amide with PhMe2SiLi and its insertion into the Si–Li bond of a second equivalent. Chem. Commun. 713–714 (1998).
Krasovskiy, A., Krasovskaya, V. & Knochel, P. Mixed Mg/Li amides of the type R2NMgCl·LiCl as highly efficient bases for the regioselective generation of functionalized aryl and heteroaryl magnesium compounds. Angew. Chem. Int. Ed. 45, 2958–2961 (2006).
Mulvey, R. E. Avant-garde metalating agents: structural basis of alkali-metal-mediated metalation. Acc. Chem. Res. 42, 743–755 (2009).
Mulvey, R. E. Modern ate chemistry: applications of synergic mixed alkali-metal−magnesium or −zinc reagents in synthesis and structure building. Organometallics 25, 1060–1075 (2006).
Kennedy, A. R., Klett, J., Mulvey, R. E. & Wright, D. S. Synergic sedation of sensitive anions: alkali-mediated zincation of cyclic ethers and ethene. Science 326, 706–708 (2009).
Blair, V. L., Kennedy, A. R., Klett, J. & Mulvey, R. E. Structural complexity of the magnesiation of furan: an octadecanuclear product with a subporphyrin-like Mg3(2,5-fur-di-yl)3 substructure. Chem. Commun. 5426–5428 (2008).
Carrella L. M. et al. Sodium-mediated manganation: direct mono- and dimanganation of benzene and synthesis of a transition-metal inverse-crown complex. Angew. Chem. Int. Ed. 46, 4662–4666 (2007).
Kennedy, A. R., MacLellan, J. G. & Mulvey, R. E. A new Na/Mg inverse crown ether. Acta Cryst. C59, m302–m303 (2003).
Kveseth, K., Seip, R. & Kohl, D. A. Conformational analysis. The structure and torsional potential of 1,3-butadiene as studied by gas electron diffraction. Acta Chem. Scand. A34, 31–42 (1980).
Caminati, W., Grassi, G. & Bauder, A. Microwave Fourier transform spectrum of s-trans-1,3-butadiene-1,1-d2 . Chem. Phys. Lett. 148, 13–16 (1988).
Churchill, M. R. & Wormald, J. The preparation and crystallographic characterization of trans-1,4-bis(dicarbonyl-π-cyclopentadienyliron)buta-1,3-diene: a complex with an anomalous proton magnetic resonance spectrum. Chem. Commun. 1217–1218 (1968).
Campbell, C. H. & Green, M. L. H. Evidence for a 1,3-sigmatropic shift in a fluxional organometallic system, trans,trans-1,4-bis(dicarbonyl-π-cyclopentadienyliron)buta-1,3-diene: nuclear magnetic resonance study. Chem. Commun. 1009–1010 (1970).
Anet, F. A. L. & Abrams, O. J. The absence of valence tautomerism in trans,trans-1,4-bis(dicarbonyl-π-cyclopentadienyliron)buta-1,3-diene. Chem. Commun. 1611–1612 (1970).
Kennedy, A. R., Klett, J., Mulvey, R. E., Newton, S. & Wright, D. S. Manganese(ii)–lithium and –sodium inverse crown ether (ICE) complexes. Chem. Commun. 308–310 (2008).
Aspinall, H. C. & Tillotson, M. R. Rare earth azatrane chemistry: facile cleavage of THF to give a Y2Li3O cluster. Inorg. Chem. 35, 2163–2164 (1996).
Clegg, W. et al. Regioselective tetrametalation of ferrocene in a single reaction: Extension of s-block inverse crown chemistry to the d-block. Angew. Chem. Int. Ed. 40, 3902–3905 (2001).
Acknowledgements
This work was supported by the UK Engineering and Physical Science Research Council. R.E.M. thanks the Royal Society/Wolfson Foundation for a research merit award. J.K. thanks the Royal Society of Edinburgh/BP Trust for a research Fellowship. We also thank J.A. Parkinson for advice on the NMR spectroscopic experiments.
Author information
Authors and Affiliations
Contributions
R.E.M. conceived the project and wrote the manuscript. J.K. added ideas and designed and performed some experiments. V.L.B. performed most syntheses and spectroscopic studies. W.C., A.R.K. and L.R. carried out X-ray crystallographic work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1125 kb)
Supplementary information
Crystallographic information for compound 4 (CIF 13 kb)
Supplementary information
Crystallographic information for compound 5 (CIF 29 kb)
Supplementary information
Crystallographic information for compound 6 (CIF 34 kb)
Rights and permissions
About this article
Cite this article
Mulvey, R., Blair, V., Clegg, W. et al. Cleave and capture chemistry illustrated through bimetallic-induced fragmentation of tetrahydrofuran. Nature Chem 2, 588–591 (2010). https://doi.org/10.1038/nchem.667
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.667
This article is cited by
-
Opportunities with calcium Grignard reagents and other heavy alkaline-earth organometallics
Nature Reviews Chemistry (2023)
-
Direct C–H metallation of tetrahydrofuran and application in flow
Nature Synthesis (2022)
-
Deconstructing THF
Nature Chemistry (2010)