Abstract
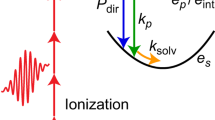
Solvated electrons in liquid water are one of the seemingly simplest, but most important, transients in chemistry and biology, but they have resisted disclosing important information about their energetics, binding motifs and dynamics. Here we report the first ultrafast liquid-jet photoelectron spectroscopy measurements of solvated electrons in liquid water. The results prove unequivocally the existence of solvated electrons bound at the water surface and of solvated electrons in the bulk solution, with vertical binding energies of 1.6 eV and 3.3 eV, respectively, and with lifetimes longer than 100 ps. The unexpectedly long lifetime of solvated electrons bound at the water surface is attributed to a free-energy barrier that separates surface and interior states. Beyond constituting important energetic and kinetic benchmark and reference data, the results also help to understand the mechanisms of a number of very efficient electron-transfer processes in nature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Boag, J. W. & Hart, E. J. Absorption spectra in irradiated water and some solutions – absorption spectra of hydrated electrons. Nature 197, 45–47 (1963).
Baletto, F., Cavazzoni, C. & Scandolo, S. Surface trapped excess electrons on ice. Phys. Rev. Lett. 95, 176801 (2005).
Boudaiffa, B. et al. Resonant formation of DNA strand breaks by low-energy (3 to 20 eV) electrons. Science 287, 1658–1660 (2000).
Duncan Lyngdoh, R. H. & Schaefer, H. F. Elementary lesions in DNA subunits: electron, hydrogen atom, proton, and hydride transfers. Acc. Chem. Res. 42, 563–572 (2009).
Gu, J., Xie, Y. & Schaefer, H. F. Electron attachment to nucleotides in aqueous solution. Chem. Phys. Chem. 7, 1885–1887 (2006).
Lin, J. P., Balabin, I. A. & Beratan, D. N. The nature of aqueous tunneling pathways between electron-transfer proteins. Science 310, 1311–1313 (2005).
Lu, Q.-B. & Sanche, L. Effects of cosmic rays on atmospheric chlorofluorocarbon dissociation and ozone depletion. Phys. Rev. Lett. 87, 078501 (2001).
Sanche, L. Beyond radical thinking. Nature 461, 358–359 (2009).
Wang, C.-R. et al. Resonant dissociative electron transfer of the presolvated electron to CCl4 in liquid: direct observation and lifetime of the CCl4*− transition state. J. Chem. Phys. 128, 041102 (2008).
Wang, C.-R., Nguyen, J. & Lu, Q.-B. Bond breaks of nucleotides by dissociative electron transfer of nonequilibrium prehydrated electrons: a new molecular mechanism for reductive DNA damage. J. Am. Chem. Soc. 131, 11320–11322 (2009).
Simons, J. How do low-energy (0.1–2 eV) electrons cause DNA-strand breaks? Acc. Chem. Res. 39, 772–779 (2006).
Buxon, G. V., Greenstock, C. L., Helman, W. P. & Ross, A. B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J. Phys. Chem. Ref. Data 17, 513–886 (1988).
Faubel, M. & Kisters, T. Non-equilibrium molecular evaporation of carboxylic-acid dimers. Nature 339, 527–529 (1989).
Faubel, M., Schlemmer, S. & Toennies, J. P. A molecular-beam study of the evaporation of water from a liquid jet. Z. Phys. D 10, 269–277 (1988).
Aziz, E. F. et al. Interaction between liquid water and hydroxide revealed by core--hole de-excitation. Nature 455, 89–91 (2008).
Winter, B. & Faubel, M. Photoemission from liquid aqueous solutions. Chem. Rev. 106, 1176–1211 (2006).
Link, O. et al. Ultrafast phase transition in metastable water near liquid interfaces. Faraday Discuss. 141, 67–79 (2009).
Link, O. et al. Ultrafast electronic spectroscopy for chemical analysis near liquid water interfaces: concepts and applications. Appl. Phys. A 96, 117–135 (2009).
Barnett, R. N., Landmann, U., Cleveland, C. L. & Jortner, J. Electron localization in water clusters. II. Surface and internal states. J. Chem. Phys. 88, 4429–4447 (1988).
Frigato, T. et al. Ab initio molecular dynamics simulation of a medium-sized water cluster anion: from an interior to a surface-located excess electron via a delocalized state. J. Phys. Chem. A 112, 6125–6133 (2008).
Herbert, J. M. & Head-Gordon, M. First-principles, quantum-mechanical simulations of electron solvation by water clusters. Proc. Natl Acad. Sci. USA 103, 14282–14287 (2006).
Lee, H. M., Suh, S. B. & Kim, K. S. Water heptamer with an excess electron: ab initio study. J. Chem. Phys. 118, 9981–9986 (2003).
Madarasz, A., Rossky, P. J. & Turi, L. Interior- and surface-bound excess electron states in large water cluster anions. J. Chem. Phys. 130, 124319 (2009).
Sommerfeld, T. & Jordan, K. D. Electron binding motifs of (H2O)n− clusters. J. Am. Chem. Soc. 128, 5828–5833 (2006).
Turi, L. & Borgis, D. Analytic investigations of an electron-water molecule pseudo potential. II. Development of a new pair potential and molecular dynamics simulations. J. Chem. Phys. 117, 6186–6195 (2002).
Turi, L., Sheu, W. S. & Rossky, P. J. Characterization of excess electrons in water-cluster anions by quantum simulations. Science 309, 914–917 (2005).
Ayotte, P. & Johnson, M. A. Electronic absorption spectra of size-selected hydrated electron clusters: (H2O)n−, n = 6–50. J. Chem. Phys. 106, 811–814 (1997).
Kim, J., Becker, I., Cheshnovsky, O. & Johnson, M. A. Photoelectron spectroscopy of the ‘missing’ hydrated electron clusters (H2O)n−, n = 3, 5, 8 and 9: isomers and continuity with the dominant clusters n = 6, 7 and ≥11. Chem. Phys. Lett. 297, 90–96 (1998).
Coe, J. V., Williams, S. M. & Bowen, K. H. Photoelectron spectra of hydrated electron clusters vs. cluster size: connecting to bulk. Int. Rev. Phys. Chem. 27, 27–51 (2008).
Kammrath, A., Verlet, J. R. R., Griffin, G. B. & Neumark, D. M. Photoelectron spectroscopy of large (water)n− (n = 50–200) clusters at 4.7 eV. J. Chem. Phys. 125, 076101 (2006).
Hammer, N. I. et al. Vibrational predissociation spectroscopy of the (H2O)6–21− clusters in the OH stretching region: evolution of the excess electron-binding signature into the intermediate cluster regime. J. Chem. Phys. 123, 244311 (2005).
Verlet, J. R. R. et al. Observation of large water-cluster anions with surface-bound excess electrons. Science 307, 93–96 (2005).
Bragg, A. E. et al. Hydrated electron dynamics: from clusters to bulk. Science 306, 669–671 (2004).
Paik, D. H. et al. Electrons in finite-sized water cavities: hydration dynamics observed in real time. Science 306, 672–675 (2004).
Neumark, D. M. Spectroscopy and dynamics of excess electrons in clusters. Mol. Phys. 106, 2183–2197 (2008).
Ma, L., Mayer, K., Chirot, F. & von Issendorf, B. Low temperature photoelectron spectra of water cluster anions. J. Chem. Phys. 131, 144303 (2009).
Elles, C. G., Jailaubekov, A. E., Crowell, R. A. & Bradforth, S. E. Excitation-energy dependence of the mechanism for two-photon ionization of liquid H2O and D2O from 8.3 to 12.4 eV. J. Chem. Phys. 125, 044515 (2006).
Laenen, R., Roth, T. & Laubereau, A. Novel precursors of solvated electrons in water: evidence for a charge transfer process. Phys. Rev. Lett. 85, 50–53 (2000).
Ottosson, N. et al. Photoemission spectroscopy of liquid water and aqueous solution: electron effective attenuation lengths and emission-angle anisotropy. J. Electron Spectrosc. Relat. Phenom. doi:10.1016/j.elspec.2009.08.007 (2009).
Lenchenkov, V., Kloepfer, J., Vilchiz, V. & Bradforth, S. E. Electron photodetachment from [Fe(CN)6]4−: photoelectron relaxation and geminate recombination. Chem. Phys. Lett. 342, 277–286 (2001).
Petersen, P. B. & Saykally, R. J. Adsorption of ions to the surface of dilute electrolyte solutions: the Jones–Ray effect revisited. J. Am. Chem. Soc. 127, 15446–15452 (2005).
Madarasz, A., Rossky, P. J. & Turi, L. Excess electron relaxation dynamics at water/air interfaces. J. Chem. Phys. 126, 234707 (2007).
Yokoyama, K. et al. Detailed investigation of the femtosecond pump–probe spectroscopy of the hydrated electron. J. Phys. Chem. A 102, 6957–6966 (1998).
Acknowledgements
Discussions with D. Neumark and P. Jungwirth are acknowledged. This work was supported by the Deutsche Forschungsgemeinschaft within the programmes SPP1134, GK 782 and SFB 755, the CRC (Nano-Spectroscopy and X-Ray Imaging) in Göttingen and the University of Leipzig.
Author information
Authors and Affiliations
Contributions
B.A., M.F. and B.W. designed the experiments. K.R.S., Y.L., E.L. and O.L. performed the experiments. K.R.S. and Y.L. analysed the data. K.R.S., Y.L., O.L. and E.L. contributed materials and/or analysis tools. B.A., B.W. and U.B. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Siefermann, K., Liu, Y., Lugovoy, E. et al. Binding energies, lifetimes and implications of bulk and interface solvated electrons in water. Nature Chem 2, 274–279 (2010). https://doi.org/10.1038/nchem.580
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.580
This article is cited by
-
Spectroscopy and dynamics of the hydrated electron at the water/air interface
Nature Communications (2024)
-
Theoretical investigation of Aryl/Alkyl halide reduction with hydrated electrons from energy and AIMD aspects
Journal of Molecular Modeling (2023)
-
Observation of a transient intermediate in the ultrafast relaxation dynamics of the excess electron in strong-field-ionized liquid water
Nature Communications (2022)
-
In situ monitoring of the influence of water on DNA radiation damage by near-ambient pressure X-ray photoelectron spectroscopy
Communications Chemistry (2021)
-
Molecular reactions at aqueous interfaces
Nature Reviews Chemistry (2020)