Abstract
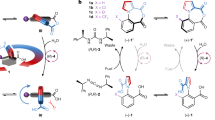
Although chemists have made small-molecule rotary motors, to date there have been no reports of small-molecule linear motors. Here we describe the synthesis and operation of a 21-atom two-legged molecular unit that is able to walk up and down a four-foothold molecular track. High processivity is conferred by designing the track-binding interactions of the two feet to be labile under different sets of conditions such that each foot can act as a temporarily fixed pivot for the other. The walker randomly and processively takes zero or one step along the track using a ‘passing-leg’ gait each time the environment is switched between acid and base. Replacing the basic step with a redox-mediated, disulfide-exchange reaction directionally transports the bipedal molecules away from the minimum-energy distribution by a Brownian ratchet mechanism. The ultimate goal of such studies is to produce artificial, linear molecular motors that move directionally along polymeric tracks to transport cargoes and perform tasks in a manner reminiscent of biological motor proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schliwa, M. (ed.) Molecular Motors (Wiley-VCH, 2003).
Kelly, T. R., De Silva, H. & Silva, R. A. Unidirectional rotary motion in a molecular system. Nature 401, 150–152 (1999).
Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
Leigh, D. A., Wong, J. K. Y., Dehez, F. & Zerbetto, F. Unidirectional rotation in a mechanically interlocked molecular rotor. Nature 424, 174–179 (2003).
Thordarson, P., Bijsterveld, E. J. A., Rowan, A. E. & Nolte, R. J. M. Epoxidation of polybutadiene by a topologically linked catalyst. Nature 424, 915–918 (2003).
van Delden, R. A. et al. Unidirectional molecular motor on a gold surface. Nature 437, 1337–1340 (2005).
Fletcher, S. P., Dumur, F., Pollard, M. M. & Feringa, B. L. A reversible, unidirectional molecular rotary motor driven by chemical energy. Science 310, 80–82 (2005).
Balzani, V. et al. Autonomous artificial nanomotor powered by sunlight. Proc. Natl Acad. Sci. USA 103, 1178–1183 (2006).
Muraoka, T., Kinbara, K. & Aida, T. Mechanical twisting of a guest by a photoresponsive host. Nature 440, 512–515 (2006).
Serreli, V., Lee, C.-F., Kay, E. R. & Leigh, D. A. A molecular information ratchet. Nature 445, 523–527 (2007).
Kay, E. R., Leigh, D. A. & Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 46, 72–191 (2007).
Sherman, W. B. & Seeman, N. C. A precisely controlled DNA biped walking device. Nano Lett. 4, 1203–1207 (2004).
Shin, J.-S. & Pierce, N. A. A synthetic DNA walker for molecular transport. J. Am. Chem. Soc. 126, 10834–10835 (2004).
Yin, P., Yan, H., Daniell, X. G., Turberfield, A. J. & Reif, J. H. A unidirectional DNA walker that moves autonomously along a track. Angew. Chem. Int. Ed. 43, 4906–4911 (2004).
Tian, Y., He, Y., Chen, Y., Yin, P. & Mao, C. A DNAzyme that walks processively and autonomously along a one-dimensional track. Angew. Chem. Int. Ed. 44, 4355–4358 (2005).
Pei, R. et al. Behavior of polycatalytic assemblies in a substrate-displaying matrix. J. Am. Chem. Soc. 128, 12693–12699 (2006).
Yin, P., Choi, H. M. T., Calvert, C. R. & Pierce, N. A. Programming biomolecular self-assembly pathways. Nature 451, 318–322 (2008).
Green, S. J., Bath, J. & Turberfield, A. J. Coordinated chemomechanical cycles: a mechanism for autonomous molecular motion. Phys. Rev. Lett. 101, 238101 (2008).
Omabegho, T., Sha, R. & Seeman, N. C. A bipedal DNA Brownian motor with coordinated legs. Science 324, 67–71 (2009).
Astumian, R. D. Design principles for Brownian molecular machines: how to swim in molasses and walk in a hurricane. Phys. Chem. Chem. Phys. 9, 5067–5083 (2007).
Astumian, R. D. & Derényi, I. Fluctuation driven transport and models of molecular motors and pumps. Eur. Biophys. J. 27, 474–489 (1998).
Rowan, S. J., Cantrill, S. J., Cousins, G. R. L., Sanders, J. K. M. & Stoddart, J. F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 41, 898–952 (2002).
Corbett, P. T. et al. Dynamic combinatorial chemistry. Chem. Rev. 106, 3652–3711 (2006).
Lehn, J.-M. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 36, 151–160 (2007).
Goral, V., Nelen, M. I., Eliseev, A. V. & Lehn, J.-M. Double-level ‘orthogonal’ dynamic combinatorial libraries on transition metal template. Proc. Natl Acad. Sci. USA 98, 1347–1352 (2001).
Orrillo, A. G., Escalante, A. M. & Furlan, R. L. E. Covalent double level dynamic combinatorial libraries: selectively addressable exchange processes. Chem. Commun. 5298–5300 (2008).
Rodriguez-Docampo, Z. & Otto, S. Orthogonal or simultaneous use of disulfide and hydrazone exchange in dynamic covalent chemistry in aqueous solution. Chem. Commun. 5301–5303 (2008).
Otto, S., Furlan, R. L. E. & Sanders, J. K. M. Dynamic combinatorial libraries of macrocyclic disulfides in water. J. Am. Chem. Soc. 122, 12063–12064 (2000).
Noji, H., Yasuda, R., Yoshida, M. & Kinosita, K. Direct observation of the rotation of F1-ATPase. Nature 386, 299–302 (1997).
Vale, R. D. et al. Direct observation of single kinesin molecules moving along microtubules. Nature 380, 451–453 (1996).
Case, R. B., Pierce, D. W., Hom-Booher, N., Hart, C. L. & Vale, R. D. The directional preference of kinesin motors is specified by an element outside of the motor catalytic domain. Cell 90, 959–966 (1997).
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EPSRC) National Mass Spectrometry Service Centre (Swansea, UK) for high-resolution mass spectrometry and Juraj Bella for assistance with high-field NMR spectroscopy. This research was funded through the European Research Council Advanced Grant WalkingMols. D.A.L. is an EPSRC Senior Research Fellow and holds a Royal Society–Wolfson Research Merit Award.
Author information
Authors and Affiliations
Contributions
M.v.D. and E.M.G. carried out the experimental work. All the authors contributed to the design of the experiments, the analysis of the data and the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2648 kb)
Rights and permissions
About this article
Cite this article
von Delius, M., Geertsema, E. & Leigh, D. A synthetic small molecule that can walk down a track. Nature Chem 2, 96–101 (2010). https://doi.org/10.1038/nchem.481
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.481
This article is cited by
-
Selective synthesis of tightly- and loosely-twisted metallomacrocycle isomers towards precise control of helicity inversion motion
Nature Communications (2023)
-
Photogearing as a concept for translation of precise motions at the nanoscale
Nature Chemistry (2022)
-
Moving forward in the semantic soup of artificial molecular machine taxonomy
Nature Nanotechnology (2022)
-
Organic crystal-based flexible smart materials
Science China Materials (2022)
-
Thermal- and Light-driven Metathesis Reactions Between Different Diselenides
Chemical Research in Chinese Universities (2022)