Abstract
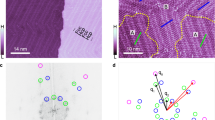
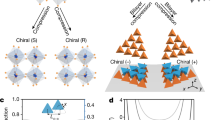
There is considerable interest in skewing the transmission of chirality, or ‘handedness’, from the molecular to the supramolecular level so that single-handed superstructures are created from mixed enantiomer systems. One approach is to flip the chirality of all the molecular building blocks to the same handedness. However, manipulation of molecular chirality is not possible for non-interconvertible enantiomers, and mechanisms that skew such systems are unclear. Here, we track the molecule-to-supramolecular chiral transfer in such systems at the nanoscale by probing molecular monolayers at surfaces. Scanning tunnelling microscopy and theoretical modelling show that enantiomeric imbalances lead to nonlinear symmetry breaking in organization, driven by configurational entropy effects. Thus, the majority enantiomer readily organizes into its superstructure with the minority left fragmented and disorganized, and thus impeded from realizing its superstructure. Such effects promise new strategies in chiral separations and enantioselective processes, and may have contributed to the homochiral evolution of complex matter from prebiotic environments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Weissbuch, I., Leiserowitz, L. & Lahav, M. Stochastic ‘mirror symmetry breaking’ via self-assembly, reactivity and amplification of chirality: relevance to abiotic conditions. Top. Curr. Chem. 259, 123–165 (2005).
Jacques, J., Collet, A. & Wilen S. H. Enantiomers, Racemates and Resolutions (Krieger, 1994).
Green, M. M. et al. Macromolecule stereochemistry: the out-of-proportion influence of optically active comonomers on the conformational characteristics of polyisocyanates. The sergeants and soldiers experiment. J. Am. Chem. Soc. 111, 6452–6454 (1989).
Sakai, K., Hirayama, N. & Tamura, R. Novel Optical Resolution Technologies (Springer, 2007).
Barlow, S. M. & Raval, R. Complex organic molecules at metal surfaces: bonding, organisation and chirality. Surf. Sci. Rep. 50, 201–341 (2003).
Humblot, V., Barlow, S. M. & Raval, R. Two-dimensional organisational chirality through supramolecular assembly of molecules at metal surfaces. Prog. Surf. Sci. 76, 1–19 (2004).
Fasel, R., Parschau, M. & Ernst, K. H. Amplification of chirality in two-dimensional enantiomorphous lattices. Nature 439, 449–452 (2006).
Parschau, M., Romer, S. & Ernst, K. H. Induction of homochirality in achiral enantiomorphous monolayers. J. Am. Chem. Soc. 126, 15398–15399 (2004).
Parschau, M., Kampen, T. & Ernst, K. H. Homochirality in monolayers of achiral meso tartaric acid. Chem. Phys. Lett. 407, 433–437 (2005).
Perez-Garcia, L. & Amabilino, D. B. Spontaneous resolution, whence and whither: from enantiomorphic solids to chiral liquid crystals, monolayers and macro- and supra-molecular polymers and assemblies. Chem. Soc. Rev. 36, 941–967 (2007).
de Feyter, S. & de Schryver, F. C. Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem. Soc. Rev. 32, 139–150 (2003).
Rankin, R. B. & Sholl, D. S. Structures of glycine, enantiopure alanine, and racemic alanine adlayers on Cu(110) and Cu(100) surfaces. J. Phys. Chem. B 109, 16764–16773 (2005).
Weigelt, S. et al. Chiral switching by spontaneous conformational change in adsorbed organic molecules. Nature Mater. 5, 112–117 (2006).
Blum, M. C., Cavar, E., Pivetta, M., Patthey, F. & Schneider, W. D. Conservation of chirality in a hierarchical supramolecular self-assembled structure with pentagonal symmetry. Angew. Chem. Int. Ed. 44, 5334–5337 (2005).
Lavoie, S., Laliberte, M. A., Temprano, I. & McBreen, P. H. A generalized two-point H-bonding model for catalytic stereoselective hydrogenation of activated ketones on chirally modified platinum. J. Am. Chem. Soc. 128, 7588–7593 (2006).
Liu, N., Haq, S., Darling, G. R. & Raval, R. Direct visualization of enantiospecific substitution of chiral guest molecules into heterochiral molecular assemblies at surfaces. Angew. Chem. Int. Ed. 46, 7613–7616 (2007).
Chen, Q. & Richardson, N. V. Enantiomeric interactions between nucleic acid bases and amino acids on solid surfaces. Nature Mater. 2, 324–328 (2003).
Lingenfelder, M. et al. Tracking the chiral recognition of adsorbed dipeptides at the single-molecule level. Angew. Chem. Int. Ed. 46, 4492–4495 (2007).
Switzer, J. A., Kothari, H. M., Poizot, P., Nakanishi, S. & Bohannan, E. W. Enantiospecific electrodeposition of a chiral catalyst. Nature 425, 490–493 (2003).
Lorenzo, M. O., Baddeley, C. J., Muryn, C. & Raval, R. Extended surface chirality from supramolecular assemblies of adsorbed chiral molecules. Nature 404, 376–379 (2000).
Lorenzo, M. O. et al. Creating chiral surfaces for enantioselective heterogeneous catalysis: R,R-tartaric acid on Cu(110). J. Phys. Chem. B 103, 10661–10669 (1999).
Lorenzo, M. O. et al. Chemical transformations, molecular transport, and kinetic barriers in creating the chiral phase of (R,R)-tartaric acid on Cu(110). J. Catal. 205, 123–134 (2002).
Fasel, R., Wider, J., Quitmann, C., Ernst, K. H. & Greber, T. Determining the absolute chirality of adsorbed molecules. Angew. Chem. Int. Ed. 43, 2853–2856 (2004).
Barbosa, L. M. M. & Sautet, P. Stability of chiral domains produced by adsorption of tartaric acid isomers on the Cu(110) surface: a periodic density functional theory study. J. Am. Chem. Soc. 123, 6639–6648 (2001).
Hermse, C. G. M. et al. Formation of chiral domains for tartaric acid on Cu(110): a combined DFT and kinetic Monte Carlo study. J. Phys. Chem. B 108, 11035–11043 (2004).
Pasteur, L. Recherches sur les relations qui peuvent exister entre la forme cristalline, la composition chimique et le sens de la polarisation rotatoire. Ann. Chim. Phys. 24, 442–449 (1848).
Kondepudi, D. K. & Crook, K. E. Theory of conglomerate crystallisation in the presence of chiral impurities. Cryst. Growth Design 5, 2173–2179 (2005).
Kellogg, R. M., Kaptein, B. & Vries, T. R. Dutch resolution of racemates and the roles of solid solution formation and nucleation inhibition. Top. Curr. Chem. 269, 159–198 (2007).
Coquerel, G. Preferential crystallisation. Top. Curr. Chem. 269, 1–51 (2007).
Green, M. M. et al. Majority rules in the copolymerisation of mirror image isomers. J. Am. Chem. Soc. 117, 4181–4182 (1995).
van Gestel, J., Palmans, A. R. A., Titulaer, B., Vekemans, J. A. J. M. & Meijer, E.W. ‘Majority-rules’ operative in chiral columnar stacks of C3-symmetrical molecules. J. Am. Chem. Soc. 127, 5490–5494 (2005).
Avalos, M., Babiano, R., Cintas, P., Jimenez, J. L. & Palacios, J. C. Symmetry breaking by spontaneous crystallization—is it the most plausible source of terrestial handedness we have long been looking for?—A reappraisal. Orig. Life Evol. Bios. 34, 391–405 (2004).
Acknowledgements
We are thankful for grants from Engineering and Physical Sciences Research Council, Biotechnology and Biological Sciences Research Council, European Union Marie Curie Research Training Networks CHEXTAN and the European Union Seventh Framework small-scale collaborative project RESOLVE.
Author information
Authors and Affiliations
Contributions
S.H, N.L and V.H. performed the experiments and analysed the data. A.P.J.J. carried out the theoretical modelling and analysis and co-wrote this section. R.R. conceived and designed the experiments, analysed the data and wrote the paper.
Corresponding author
Supplementary information
Supplementary information
Supplementary information (PDF 466 kb)
Rights and permissions
About this article
Cite this article
Haq, S., Liu, N., Humblot, V. et al. Drastic symmetry breaking in supramolecular organization of enantiomerically unbalanced monolayers at surfaces. Nature Chem 1, 409–414 (2009). https://doi.org/10.1038/nchem.295
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.295
This article is cited by
-
Chirality control of a single carbene molecule by tip-induced van der Waals interactions
Nature Communications (2023)
-
Symmetry Breaking by Consecutive Amplification: Efficient Paths to Homochirality
Origins of Life and Evolution of Biospheres (2022)
-
Simultaneous switching of supramolecular chirality and organizational chirality driven by Coulomb expansion
Nano Research (2022)
-
Homochirality in biomineral suprastructures induced by assembly of single-enantiomer amino acids from a nonracemic mixture
Nature Communications (2019)
-
Amplification of chirality in surface-confined supramolecular bilayers
Nature Communications (2018)