Abstract
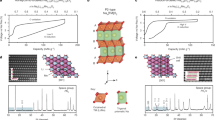
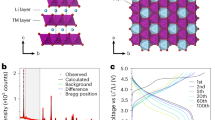
The search for improved energy-storage materials has revealed Li- and Na-rich intercalation compounds as promising high-capacity cathodes. They exhibit capacities in excess of what would be expected from alkali-ion removal/reinsertion and charge compensation by transition-metal (TM) ions. The additional capacity is provided through charge compensation by oxygen redox chemistry and some oxygen loss. It has been reported previously that oxygen redox occurs in O 2p orbitals that interact with alkali ions in the TM and alkali-ion layers (that is, oxygen redox occurs in compounds containing Li+–O(2p)–Li+ interactions). Na2/3[Mg0.28Mn0.72]O2 exhibits an excess capacity and here we show that this is caused by oxygen redox, even though Mg2+ resides in the TM layers rather than alkali-metal (AM) ions, which demonstrates that excess AM ions are not required to activate oxygen redox. We also show that, unlike the alkali-rich compounds, Na2/3[Mg0.28Mn0.72]O2 does not lose oxygen. The extraction of alkali ions from the alkali and TM layers in the alkali-rich compounds results in severely underbonded oxygen, which promotes oxygen loss, whereas Mg2+ remains in Na2/3[Mg0.28Mn0.72]O2, which stabilizes oxygen.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Whittingham, M. S. Lithium batteries and cathode materials. Chem. Rev. 104, 4271–4302 (2004).
Croguennec, L. & Palacin, M. R. Recent achievements on inorganic electrode materials for lithium-ion batteries. J. Am. Chem. Soc. 137, 3140–3156 (2015).
Larcher, D. & Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2015).
Seo, D.-H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Lu, Z. & Dahn, J. R. The effect of Co substitution for Ni on the structure and electrochemical behavior of T2 and O2 structure Li2/3 [CoxNi1/3–xMn2/3]O2 . J. Electrochem. Soc. 148, A237 (2001).
Kim, J.-S. et al. Electrochemical and structural properties of xLi2M′O3 (1−x)LiMn0.5Ni0.5O2 electrodes for lithium batteries (M′ = Ti, Mn, Zr; 0 ⩽ x ⩽ 0.3). Chem. Mater. 16, 1996–2006 (2004).
Koga, H. et al. Different oxygen redox participation for bulk and surface: a possible global explanation for the cycling mechanism of Li1.20Mn0.54Co0.13Ni0.13O2 . J. Power Sources 236, 250–258 (2013).
Luo, K. et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 8, 684–691 (2016).
Perez, A. J. et al. Strong oxygen participation in the redox governing the structural and electrochemical properties of Na-rich layered oxide Na2IrO3 . Chem. Mater. 28, 8278–8288 (2016).
McCalla, E. et al. Visualization of O–O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516–1521 (2015).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
Saubanère, M., McCalla, E., Tarascon, J.-M. & Doublet, M.-L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 9, 984–991 (2016).
Koga, H. et al. Reversible oxygen participation to the redox processes revealed for Li1.20Mn0.54Co0.13Ni0.13O2 . J. Electrochem. Soc. 160, A786–A792 (2013).
Oishi, M. et al. Direct observation of reversible charge compensation by oxygen ion in Li-rich manganese layered oxide positive electrode material, Li1.16Ni0.15Co0.19Mn0.50O2 . J. Power Sources 276, 89–94 (2015).
Luo, K. et al. Anion redox chemistry in the cobalt free 3d transition-metal oxide intercalation electrode Li[Li0.2Ni0.2Mn0.6]O2 . J. Am. Chem. Soc. 138, 11211–11218 (2016).
Rozier, P. et al. Anionic redox chemistry in Na-rich Na2Ru1−ySnyO3 positive electrode material for Na-ion batteries. Electrochem. Commun. 53, 29–32 (2015).
Du, K. et al. Exploring reversible oxidation of oxygen in a manganese oxide. Energy Environ. Sci. 6, 3–5 (2016).
Castel, E., Berg, E. J., El Kazzi, M., Novák, P. & Villevieille, C. Differential electrochemical mass spectrometry study of interface of xLi2MnO3·(1−x)LiMO2 (M = Ni, Co, Mn) material as positive electrode in Li-ion batteries. Chem. Mater. 26, 5051–5057 (2014).
Yabuuchi, N. et al. A new electrode material for rechargeable sodium batteries: P2-type Na2/3[Mg0.28Mn0.72]O2 with anomalously high reversible capacity. J. Mater. Chem. A 2, 16851–16855 (2014).
Freire, M. et al. A new active Li–Mn–O compound for high energy density Li-ion batteries. Nat. Mater. 15, 173–177 (2015).
Meng, Y. S. et al. Cation ordering in layered O3 Li[NixLi1/3−2x/3Mn2/3−x/3]O2 (0 ≤ x ≤ 1/2) compounds. Chem. Mater. 17, 2386–2394 (2005).
Yabuuchi, N. et al. New O2/P2-type Li-excess layered manganese oxides as promising multi-functional electrode materials for rechargeable Li/Na batteries. Adv. Energy Mater. 4, 1301453 (2014).
Stoyanova, R. et al. Stabilization of over-stoichiometric Mn4+ in layered Na2/3MnO2 . J. Solid State Chem. 183, 1372–1379 (2010).
Zhou, T., Zhang, D., Button, T. W., Wright, A. J. & Greaves, C. Influence of cooling rate on the structure and composition of NaxCoO2 (x ∼0.65). J. Mater. Chem. 19, 1123–1128 (2009).
Sathiya, M., Hemalatha, K., Ramesha, K., Tarascon, J.-M. & Prakash, A. S. Synthesis, structure, and electrochemical properties of the layered sodium insertion cathode material: NaNi1/3Mn1/3Co1/3O2 . Chem. Mater. 24, 1846–1853 (2012).
Duffort, V., Talaie, E., Black, R. & Nazar, L. F. Uptake of CO2 in layered P2-Na0.67Mn0.5Fe0.5O2: insertion of carbonate anions. Chem. Mater. 27, 2515–2524 (2015).
Lu, Z. & Dahn, J. R. In situ X-ray diffraction study of P2-Na2/3[Ni1/3Mn2/3]O2 . J. Electrochem. Soc. 148, A1225 (2001).
Hong, J. et al. Critical role of oxygen evolved from layered Li–excess metal oxides in lithium rechargeable batteries. Chem. Mater. 24, 2692–2697 (2012).
Aurbach, D. The correlation between surface chemistry, surface morphology, and cycling efficiency of lithium electrodes in a few polar aprotic systems. J. Electrochem. Soc. 136, 3198–3205 (1989).
McCalla, E. et al. Understanding the roles of anionic redox and oxygen release during electrochemical cycling of lithium-rich layered Li4FeSbO6 . J. Am. Chem. Soc. 137, 4804–4814 (2015).
de la Llave, E. et al. Improving energy density and structural stability of manganese oxide cathodes for Na-ion batteries by structural lithium substitution. Chem. Mater. 28, 9064–9076 (2016).
Sathiya, M. et al. High performance Li2Ru1−yMnyO3 (0.2 ≤ y ≤ 0.8) cathode materials for rechargeable lithium-ion batteries: their understanding. Chem. Mater. 25, 1121–1131 (2013).
Armstrong, A. R. et al. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2 . J. Am. Chem. Soc. 128, 8694–8698 (2006).
Lee, K. T. K. T. et al. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Chem. Mater. 26, 820–829 (2014).
Mortemard De Boisse, B., Carlier, D., Guignard, M., Bourgeois, L. & Delmas, C. P2-NaxMn1/2Fe1/2O2 phase used as positive electrode in Na batteries: structural changes induced by the electrochemical (de)intercalation process. Inorg. Chem. 53, 11197–11205 (2014).
Jiang, M., Key, B., Meng, Y. S. & Grey, C. P. Electrochemical and structural study of the layered, ‘Li-excess’ lithium-ion battery electrode material Li[Li1/9Ni1/3Mn5/9]O2 . Chem. Mater. 21, 2733–2745 (2009).
Xu, J. et al. Identifying the critical role of Li substitution in P2-Nax[LiyNizMn1−y−z]O2 (0 < x, y, z < 1) intercalation cathode materials for high-energy Na-ion batteries. Chem. Mater. 26, 1260–1269 (2014).
Enkovaara, J. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21 (2009).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77 (1996).
Schlipf, M. & Gygi, F. Optimization algorithm for the generation of ONCV pseudopotentials. Comput. Phys. Commun. 196, 36–44 (2015).
Cococcioni, M. & de Gironcoli, S. Linear response approach to the calculation of the effective interaction parameters in the LDA+ U method. Phys. Rev. B 71, 035105 (2005).
Lee, D. H., Xu, J. & Meng, Y. S. An advanced cathode for Na-ion batteries with high rate and excellent structural stability. Phys. Chem. Chem. Phys. 15, 3304–3312 (2013).
Ghiringhelli, G., Braicovich, L., Schmitt, T. & Strocov, V. High-resolution RIXS with the SAXES spectrometer at the ADRESS beamline of the Swiss Light Source. Synchrotron Radiat. News 25, 16–22 (2012).
Strocov, V. N. et al. High-resolution soft X-ray beamline ADRESS at the Swiss Light Source for resonant inelastic X-ray scattering and angle-resolved photoelectron spectroscopies. J. Synchrotron Radiat. 17, 631–643 (2010).
Ghiringhelli, G. et al. SAXES, a high resolution spectrometer for resonant x-ray emission in the 400-1600 eV energy range. Rev. Sci. Instrum. 77, 113108 (2006).
Acknowledgements
P.G.B. is indebted to the Engineering and Physical Sciences Research Council (EPSRC), including the SUPERGEN program, for financial support. We additionally thank the EPSRC for grant EP/K040375/1 for the ‘South of England Analytical Electron Microscope’. The authors thank N. Kumar, Max Planck Institute of Chemical Physics, for help with magnetic measurements. Synchrotron radiation experiments were performed at the ADRESS beamline of the Swiss Light Source at the Paul Scherrer Institute, Switzerland. We acknowledge technical and experimental support at the ADRESS beamline by L. Nue and M. Dantz. Part of this research was funded by the Swiss National Science Foundation through the Sinergia network Mott Physics Beyond the Heisenberg model and the NCCR MARVEL. The research leading to these results received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under Grant Agreement no. 290605 (CO-FUND: PSIFELLOW). The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, US Department of Energy, under Contract no. DE-AC02-05CH11231. The authors are also grateful to G. Cibin for contributing to the collection of hard XAS data.
Author information
Authors and Affiliations
Contributions
U.M., R.A.H. and M.R.R. contributed to all aspects of the research. L.C.D., N.G., J.W.S., F.M., K.L., R.H., L.J., U.M., D.E.M., X.L. and T.S. contributed to the measurement of the SXAS and RIXS spectroscopy. D.M.P., N.T.-R., S.R. and A.V.C. contributed to the data collection and analysis of hard XAS measurements. J.W.S., M.A.P.-O. and F.G. performed the DFT calculations. J.G.L. collected, processed and interpreted the STEM images. P.G.B., U.M., R.A.H., J.W.S., M.R.R. and L.C.D. interpreted the data. P.G.B. wrote the paper with contributions from U.M., R.A.H. and M.R.R. The project was supervised by P.G.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2110 kb)
Rights and permissions
About this article
Cite this article
Maitra, U., House, R., Somerville, J. et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nature Chem 10, 288–295 (2018). https://doi.org/10.1038/nchem.2923
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2923
This article is cited by
-
Sustainable layered cathode with suppressed phase transition for long-life sodium-ion batteries
Nature Sustainability (2024)
-
Hollow Na0.62K0.05Mn0.7Ni0.2Co0.1O2 polyhedra with exposed stable {001} facets and K riveting for sodium-ion batteries
Science China Materials (2023)
-
Effects of elemental doping on phase transitions of manganese-based layered oxides for sodium-ion batteries
Science China Materials (2023)
-
Understanding voltage hysteresis and decay during anionic redox reaction in layered transition metal oxide cathodes: A critical review
Nano Research (2023)
-
Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage
Nature Reviews Materials (2022)