Abstract
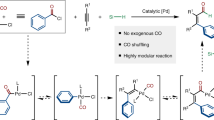
The development of metal-catalysed methods to functionalize inert C–H bonds has become a dominant research theme in the past decade as an approach to efficient synthesis. However, the incorporation of carbon monoxide into such reactions to form valuable ketones has to date proved a challenge, despite its potential as a straightforward and green alternative to Friedel–Crafts reactions. Here we describe a new approach to palladium-catalysed C–H bond functionalization in which carbon monoxide is used to drive the generation of high-energy electrophiles. This offers a method to couple the useful features of metal-catalysed C–H functionalization (stable and available reagents) and electrophilic acylations (broad scope and selectivity), and synthesize ketones simply from aryl iodides, CO and arenes. Notably, the reaction proceeds in an intermolecular fashion, without directing groups and at very low palladium-catalyst loadings. Mechanistic studies show that the reaction proceeds through the catalytic build-up of potent aroyl triflate electrophiles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Olah, G. A. Friedel–Crafts and Related Reactions (Interscience, 1963).
Sartori, G. & Maggi, R. Advances in Friedel–Crafts Acylation Reactions: Catalytic and Green Processes (CRC, 2010).
Boursalian, G. B., Ham, W. S., Mazzotti, A. R. & Ritter, T. Charge-transfer-directed radical substitution enables para-selective C–H functionalization. Nat. Chem. 8, 810–815 (2016).
Bähr, S. & Oestreich, M. Electrophilic aromatic substitution with silicon electrophiles: catalytic Friedel–Crafts C−H silylation. Angew. Chem. Int. Ed. 56, 52–59 (2017).
Vekariya, R. H. & Aubé, J. Hexafluoro-2-propanol-promoted intermolecular Friedel–Crafts acylation reaction. Org. Lett. 18, 3534–3537 (2016).
Liu, Y., Meng, G., Liu, R. & Szostak, M. Sterically-controlled intermolecular Friedel–Crafts acylation with twisted amides via selective N–C cleavage under mild conditions. Chem. Commun. 52, 6841–6844 (2016).
Hartwig, J. F. Evolution of C–H bond functionalization from methane to methodology. J. Am. Chem. Soc. 138, 2–24 (2015).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C−H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C−C bond formation via heteroatom-directed C−H bond activation. Chem. Rev. 110, 624–655 (2010).
Chen, X., Engle, K. M., Wang, D.-H. & Yu, J.-Q. Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 48, 5094–5115 (2009).
Liu, C. et al. Oxidative coupling between two hydrocarbons: an update of recent C–H functionalizations. Chem. Rev. 115, 12138–12204 (2015).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist's toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Kuhl, N., Hopkinson, M. N., Wencel-Delord, J. & Glorius, F. Beyond directing groups: transition-metal-catalyzed C–H activation of simple arenes. Angew. Chem. Int. Ed. 51, 10236–10254 (2012).
Hartwig, J. F. & Larsen, M. A. Undirected, homogeneous C–H bond functionalization: challenges and opportunities. ACS Cent. Sci. 2, 281–292 (2016).
Jia, C., Kitamura, T. & Fujiwara, Y. Catalytic functionalization of arenes and alkanes via C−H bond activation. Acc. Chem. Res. 34, 633–639 (2001).
Liu, Q., Zhang, H. & Lei, A. Oxidative carbonylation reactions: organometallic compounds (R–M) or hydrocarbons (R–H) as nucleophiles. Angew. Chem. Int. Ed. 50, 10788–10799 (2011).
Bai, Y., Davis, D. C. & Dai, M. Natural product synthesis via palladium-catalyzed carbonylation. J. Org. Chem. 82, 2319–2328 (2017).
Yang, L. & Huang, H. Transition-metal-catalyzed direct addition of unactivated C–H bonds to polar unsaturated bonds. Chem. Rev. 115, 3468–3517 (2015).
Willcox, D. et al. A general catalytic β-C–H carbonylation of aliphatic amines to β-lactams. Science 354, 851–857 (2016).
Li, S., Chen, G., Feng, C.-G., Gong, W. & Yu, J.-Q. Ligand-enabled γ-C–H olefination and carbonylation: construction of β-quaternary carbon centers. J. Am. Chem. Soc. 136, 5267–5270 (2014).
Campo, M. A. & Larock, R. C. Synthesis of fluoren-9-ones by the palladium-catalyzed cyclocarbonylation of o-halobiaryls. J. Org. Chem. 67, 5616–5620 (2002).
Wu, X.-F., Anbarasan, P., Neumann, H. & Beller, M. Palladium-catalyzed carbonylative C–H activation of heteroarenes. Angew. Chem. Int. Ed. 49, 7316–7319 (2010).
Lian, Z., Friis, S. D. & Skrydstrup, T. C–H activation dependent Pd-catalyzed carbonylative coupling of (hetero)aryl bromides and polyfluoroarenes. Chem. Commun. 51, 1870–1873 (2015).
Zhang, H. et al. Palladium-catalyzed oxidative double C–H functionalization/carbonylation for the synthesis of xanthones. Angew. Chem. Int. Ed. 51, 5204–5207 (2012).
Cernak, T. A. & Lambert, T. H. Multicatalytic synthesis of α-pyrrolidinyl ketones via a tandem palladium(II)/indium(III)-catalyzed aminochlorocarbonylation/Friedel−Crafts acylation reaction. J. Am. Chem. Soc. 131, 3124–3125 (2009).
Zeng, F. & Alper, H. Pd-catalyzed direct coupling of indoles with carbon monoxide and alkynes: selective synthesis of linear α,β-unsaturated ketones. Org. Lett. 15, 2034–2037 (2013).
Wu, X.-F., Neumann, H. & Beller, M. Palladium-catalyzed carbonylative coupling reactions between Ar–X and carbon nucleophiles. Chem. Soc. Rev. 40, 4986–5009 (2011).
Blangetti, M., Rosso, H., Prandi, C., Deagostino, A. & Venturello, P. Suzuki–Miyaura cross-coupling in acylation reactions, scope and recent developments. Molecules 18, 1188–1213 (2013).
Kuniyasu, H. et al. σ-Bond metathesis between M–X and RC(O)X′ (M = Pt, Pd; X, X′ = Cl, Br, I): facile determination of the relative ΔG values of the oxidative additions of RC(O)X to an M(0) complex, evidence by density functional theory calculations, and synthetic applications. Organometallics 32, 2026–2032 (2013).
Beller, M. & Wu, X. F. Transition Metal Catalyzed Carbonylations – Carbonylative Activation of C–X bonds (Springer, 2013).
Grigg, R. & Mutton, S. P. Pd-catalysed carbonylations: versatile technology for discovery and process chemists. Tetrahedron 66, 5515–5548 (2010).
Luo, Y.-R. Handbook of Bond Dissociation Energies in Organic Compounds (CRC, 2003).
Quesnel, J. S. & Arndtsen, B. A. A palladium-catalyzed carbonylation approach to acid chloride synthesis. J. Am. Chem. Soc. 135, 16841–16844 (2013).
Quesnel, J. S., Kayser, L. V., Fabrikant, A. & Arndtsen, B. A. Acid chloride synthesis by the palladium-catalyzed chlorocarbonylation of aryl bromides. Chem. Eur. J. 21, 9550–9555 (2015).
Tjutrins, J. & Arndtsen, B. A. An electrophilic approach to the palladium-catalyzed carbonylative C–H functionalization of heterocycles. J. Am. Chem. Soc. 137, 12050–12054 (2015).
Fang, X., Cacherat, B. & Morandi, B. CO- and HCl-free synthesis of acid chlorides from unsaturated hydrocarbons via shuttle catalysis. Nat. Chem. 9, 1105–1109 (2017).
Effenberger, F., Eberhard, J. K. & Maier, A. H. The first unequivocal evidence of the reacting electrophile in aromatic acylation reactions. J. Am. Chem. Soc. 118, 12572–12579 (1996).
Quesnel, J. S. et al. Computational study of the palladium-catalyzed carbonylative synthesis of aromatic acid chlorides: the synergistic effect of PtBu3 and CO on reductive elimination. Chem. Eur. J. 22, 15107–15118 (2016).
Lee, S. Y. & Hartwig, J. F. Palladium-catalyzed, site-selective direct allylation of aryl C–H bonds by silver-mediated C–H activation: a synthetic and mechanistic investigation. J. Am. Chem. Soc. 138, 15278–15284 (2016).
Whitaker, D., Burés, J. & Larrosa, I. Ag(I)-catalyzed C–H activation: the role of the Ag(I) salt in Pd/Ag-mediated C–H arylation of electron-deficient arenes. J. Am. Chem. Soc. 138, 8384–8387 (2016).
Lotz, M. D., Camasso, N. M., Canty, A. J. & Sanford, M. S. Role of silver salts in palladium-catalyzed arene and heteroarene C–H functionalization reactions. Organometallics 36, 165–171 (2017).
Lapointe, D. & Fagnou, K. Overview of the mechanistic work on the concerted metallation–deprotonation pathway. Chem. Lett. 39, 1118–1126 (2010).
Effenberger, F. & Maier, A. H. Changing the ortho/para ratio in aromatic acylation reactions by changing reaction conditions: a mechanistic explanation from kinetic measurements. J. Am. Chem. Soc. 123, 3429–3433 (2001).
Boller, T. M. et al. Mechanism of the mild functionalization of arenes by diboron reagents catalyzed by iridium complexes. intermediacy and chemistry of bipyridine-ligated iridium trisboryl complexes. J. Am. Chem. Soc. 127, 14263–14278 (2005).
Press, L. P., Kosanovich, A. J., McCulloch, B. J. & Ozerov, O. V. High-turnover aromatic C–H borylation catalyzed by POCOP-type pincer complexes of iridium. J. Am. Chem. Soc. 138, 9487–9497 (2016).
Obligacion, J. V., Semproni, S. P. & Chirik, P. J. Cobalt-catalyzed C–H borylation. J. Am. Chem. Soc. 136, 4133–4136 (2014).
Effenberger, F., Epple, G., Eberhard, J. K., Buehler, U. & Sohn, E. Electrophilic aromatic substitution. 24. Carboxylic trifluoromethanesulfonic and methanesulfonic anhydrides synthesis and dissociation tendency. Chem. Ber. 116, 1183–1194 (1983).
Kantor, T. G. Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacotherapy 6, 93–102 (1986).
Keating, G. M. Fenofibrate: a review of its lipid-modifying effects in dyslipidemia and its vascular effects in type 2 diabetes mellitus. Am. J. Cardiovasc. Drugs 11, 227–247 (2011).
Yin, W., Ma, Y., Xu, J. & Zhao, Y. Microwave-assisted one-pot synthesis of 1-indanones from arenes and α,β-unsaturated acyl chlorides. J. Org. Chem. 71, 4312–4315 (2006).
Acknowledgements
We thank Natural Sciences and Engineering Research Council of Canada, the Canadian Foundation for Innovation and the Centre for Green Chemistry and Catalysis (supported by Fonds de recherche du Québec – Nature et Technologies) for funding this research. We thank L. Kayser for the X-ray crystal structure of 3a and to S. Kelley for the X-ray crystal structure of 4b.
Author information
Authors and Affiliations
Contributions
B.A.A. and R.G.K. conceived the project. R.G.K. conducted the majority of the experiments. J.T. assisted in the early experiments. G.M.T. conducted the mechanistic experiments with 4b and assisted with Table 1 and Fig. 4b. N.I.L. and O.K. assisted with Table 1. B.A.A. and R.G.K. prepared the manuscript with feedback from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 8875 kb)
Supplementary information
Crystallographic data for compound 3a (CIF 657 kb)
Supplementary information
Crystallographic data for compound 4b (CIF 4930 kb)
Rights and permissions
About this article
Cite this article
Garrison Kinney, R., Tjutrins, J., Torres, G. et al. A general approach to intermolecular carbonylation of arene C–H bonds to ketones through catalytic aroyl triflate formation. Nature Chem 10, 193–199 (2018). https://doi.org/10.1038/nchem.2903
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2903
This article is cited by
-
Palladium-catalysed carboformylation of alkynes using acid chlorides as a dual carbon monoxide and carbon source
Nature Chemistry (2021)
-
In situ acyl triflates ace it
Nature Chemistry (2018)