Abstract
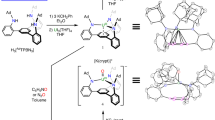
The reactivity of uranium compounds towards small molecules typically occurs through stoichiometric rather than catalytic processes. Examples of uranium catalysts reacting with water are particularly scarce, because stable uranyl groups form that preclude the recovery of the uranium compound. Recently, however, an arene-anchored, electron-rich uranium complex has been shown to facilitate the electrocatalytic formation of H2 from H2O. Here, we present the precise role of uranium–arene δ bonding in intermediates of the catalytic cycle, as well as details of the atypical two-electron oxidative addition of H2O to the trivalent uranium catalyst. Both aspects were explored by synthesizing mid- and high-valent uranium–oxo intermediates and by performing comparative studies with a structurally related complex that cannot engage in δ bonding. The redox activity of the arene anchor and a covalent δ-bonding interaction with the uranium ion during H2 formation were supported by density functional theory analysis. Detailed insight into this catalytic system may inspire the design of ligands for new uranium catalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Liddle, S. T. The renaissance of non-aqueous uranium chemistry. Angew. Chem. Int. Ed. 54, 8604–8641 (2015).
Lukens, W. L., Beshouri Sharon, M. Jr, Blosch, L. L. & Andersen, R. A. Oxidative elimination of H2 from [CP′2U(µ-OH)]2 to form [Cp′2U(µO)]2 where Cp′ is 1,3-(Me3C)2C5H3 or 1,3-(Me3Si)2C5H3 . J. Am. Chem. Soc. 118, 901–902 (1996).
Schmidt, A.-C., Heinemann, F. W., Lukens, W. W. & Meyer, K. Molecular and electronic structure of dinuclear uranium bis-μ-oxo complexes with diamond core structural motifs. J. Am. Chem. Soc. 136, 11980–11993 (2014).
Summerscales, O. T., Cloke, F. G. N., Hitchcock, P. B., Green, J. C. & Hazari, N. Reductive cyclotrimerization of carbon monoxide to the deltate dianion by an organometallic uranium complex. Science 311, 829–831 (2006).
Schmidt, A.-C., Nizovtsev, A. V., Scheurer, A., Heinemann, F. W. & Meyer, K. Uranium-mediated reductive conversion of CO2 to CO and carbonate in a single-vessel, closed synthetic cycle. Chem. Commun. 48, 8634–8636 (2012).
Halter, D. P., Heinemann, F. W., Bachmann, J. & Meyer, K. Uranium-mediated electrocatalytic dihydrogen production from water. Nature 530, 317–321 (2016).
Fox, A. R., Bart, S. C., Meyer, K. & Cummins, C. C. Towards uranium catalysts. Nature 455, 341–349 (2008).
La Pierre, H. S. & Meyer, K. Activation of small molecules by molecular uranium complexes. Prog. Inorg. Chem. 58, 303–416 (2014).
Castro-Rodriguez, I., Nakai, H., Zakharov, L. N., Rheingold, A. L. & Meyer, K. A linear, O-coordinated η1-CO2 bound to uranium. Science 305, 1757–1759 (2004).
Lam, O. P., Bart, S. C., Kameo, H., Heinemann, F. W. & Meyer, K. Insights into the mechanism of carbonate formation through reductive cleavage of carbon dioxide with low-valent uranium centers. Chem. Commun. 46, 3137–3139 (2010).
Lam, O. P. & Meyer, K. Uranium-mediated carbon dioxide activation and functionalization. Polyhedron 32, 1–9 (2012).
Schmidt, A.-C. et al. Activation of SO2 and CO2 by trivalent uranium leading to sulfite/dithionite and carbonate/oxalate complexes. Chem. Eur. J. 20, 13501–13506 (2014).
Gardner, B. M. et al. Homologation and functionalization of carbon monoxide by a recyclable uranium complex. Proc. Natl Acad. Sci. USA 109, 9265–9270 (2012).
Fortier, S., Brown, J. L., Kaltsoyannis, N., Wu, G. & Hayton, T. W. Synthesis, molecular and electronic structure of UV(O)[N(SiMe3)2]3 . Inorg. Chem. 51, 1625–1633 (2012).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Diaconescu, P. L., Arnold, P. L., Baker, T. A., Mindiola, D. J. & Cummins, C. C. Arene-bridged diuranium complexes: inverted sandwiches supported by δ backbonding. J. Am. Chem. Soc. 122, 6108–6109 (2000).
Evans, W. J., Kozimor, S. A., Ziller, J. W. & Kaltsoyannis, N. Structure, reactivity, and density functional theory analysis of the six-electron reductant, [(C5Me5)2U]2(μ-η6:η6-C6H6), synthesized via a new mode of (C5Me5)3M reactivity. J. Am. Chem. Soc. 126, 14533–14547 (2004).
Patel, D. et al. A formal high oxidation state inverse-sandwich diuranium complex a new route to f-block-metal bonds. Angew. Chem. Int. Ed. 50, 10388–10392 (2011).
Camp, C., Mougel, V., Pécaut, J., Maron, L. & Mazzanti, M. Cation-mediated conversion of the state of charge in uranium arene inverted-sandwich complexes. Chem. Eur. J. 19, 17528–17540 (2013).
Liddle, S. T. Inverted sandwich arene complexes of uranium. Coord. Chem. Rev. 293–294, 211–227 (2015).
Bart, S. C., Heinemann, F. W., Anthon, C., Hauser, C. & Meyer, K. A new tripodal ligand system with steric and electronic modularity for uranium coordination chemistry. Inorg. Chem. 48, 9419–9426 (2009).
La Pierre, H. S., Kameo, H., Halter, D. P., Heinemann, F. W. & Meyer, K. Coordination and redox isomerization in the reduction of a uranium(III) monoarene complex. Angew. Chem. Int. Ed. 53, 7154–7157 (2014).
Halter, D. P., La Pierre, H. S., Heinemann, F. W. & Meyer, K. Uranium(IV) halide (F−, Cl−, Br−, and I−) monoarene complexes. Inorg. Chem. 53, 8418–8424 (2014).
La Pierre, H. S., Scheurer, A., Heinemann, F. W., Hieringer, W. & Meyer, K. Synthesis and characterization of a uranium(II) monoarene complex supported by δ backbonding. Angew. Chem. Int. Ed. 53, 7158–7162 (2014).
Franke, S. M. et al. Uranium(III) complexes with bulky aryloxide ligands featuring metal–arene interactions and their reactivity toward nitrous oxide. Inorg. Chem. 52, 10552–10558 (2013).
Arney, D. S. J. & Burns, C. J. Synthesis and structure of high-valent organouranium complexes containing terminal monooxo functional groups. J. Am. Chem. Soc. 115, 9840–9841 (1993).
King, D. M. et al. Single-molecule magnetism in a single-ion triamidoamine uranium(V) terminal mono-oxo complex. Angew. Chem. Int. Ed. 52, 4921–4924 (2013).
Bart, S. C. et al. Carbon dioxide activation with sterically pressured mid- and high-valent uranium complexes. J. Am. Chem. Soc. 130, 12536–12546 (2008).
Batsanov, S. S. Van der Waals radii of elements. Inorg. Mater. 37, 871–885 (2001).
Effenberger, F. 1,3,5-tris(dialkylamino)benzenes: model compounds for the electrophilic substitution and oxidation of aromatic compounds. Acc. Chem. Res. 22, 27–35 (1989).
Anamimoghadam, O. et al. Electronically stabilized nonplanar phenalenyl radical and its planar isomer. J. Am. Chem. Soc. 137, 14944–14951 (2015).
Kochi, J. K., Rathore, R. & Le Maguères, P. Stable dimeric aromatic cation−radicals. Structural and spectral characterization of through-space charge delocalization. J. Org. Chem. 65, 6826–6836 (2000).
Neidig, M. L., Clark, D. L. & Martin, R. L. Covalency in f-element complexes. Coord. Chem. Rev. 257, 394–406 (2013).
Zi, G. et al. Preparation and reactions of base-free bis(1,2,4-tri-tert-butylcyclopentadienyl)uranium oxide, Cp′2UO. Organometallics 24, 4251–4264 (2005).
Kraft, S. J., Walensky, J., Fanwick, P. E., Hall, M. B. & Bart, S. C. Crystallographic evidence of a base-free uranium(IV) terminal oxo species. Inorg. Chem. 49, 7620–7622 (2010).
Evans, W. J., Kozimor, S. A. & Ziller, J. W. Bis(pentamethylcyclopentadienyl) U(III) oxide and U(IV) oxide carbene complexes. Polyhedron 23, 2689–2694 (2004).
Brown, J. L., Fortier, S., Lewis, R. A., Wu, G. & Hayton, T. W. A complete family of terminal uranium chalcogenides, [U(E)(N{SiMe3}2)3]− (E = O, S, Se, Te). J. Am. Chem. Soc. 134, 15468–15475 (2012).
Gourier, D., Caurant, D., Berthet, J. C., Boisson, C. & Ephritikhine, M. Influence of the nature of the ligands on the electronic ground state of organouranium(V) compounds, studied by electron paramagnetic resonance. Inorg. Chem. 36, 5931–5936 (1997).
Pankhurst, J. R. et al. Inner-sphere vs. outer-sphere reduction of uranyl supported by a redox-active, donor-expanded dipyrrin. Chem. Sci. 8, 108–116 (2017).
Kindra, D. R. & Evans, W. J. Magnetic susceptibility of uranium complexes. Chem. Rev. 114, 8865–8882 (2014).
Kosog, B., Kefalidis, C. E., Heinemann, F. W., Maron, L. & Meyer, K. Uranium(III)-mediated C–C-coupling of terminal alkynes: formation of dinuclear uranium(IV) vinyl complexes. J. Am. Chem. Soc. 134, 12792–12797 (2012).
Lam, O. P., Heinemann, F. W. & Meyer, K. A new diamantane functionalized tris(aryloxide) ligand system for small molecule activation chemistry at reactive uranium complexes. C. R. Chim. 13, 803–811 (2010).
Graves, C. R. et al. Probing the chemistry, electronic structure and redox energetics in organometallic pentavalent uranium complexes. Inorg. Chem. 47, 11879–11891 (2008).
Tsoureas, N., Kilpatrick, A. F. R., Inman, C. J. & Cloke, F. G. N. Steric control of redox events in organo-uranium chemistry: synthesis and characterisation of U(v) oxo and nitrido complexes. Chem. Sci. 7, 4624–4632 (2016).
Rosenzweig, M. W. et al. Uranium(IV) terminal hydrosulfido and sulfido complexes insights into the nature of the uranium–sulfur bond. Chem. Sci. 7, 5857–5866 (2016).
Anderson, N. H. et al. Harnessing redox activity for the formation of uranium tris(imido) compounds. Nat. Chem. 6, 919–926 (2014).
Mohammad, A., Cladis, D. P., Forrest, W. P., Fanwick, P. E. & Bart, S. C. Reductive heterocoupling mediated by Cp*2U(2,2′-bpy). Chem. Commun. 48, 1671–1673 (2012).
Kraft, S. J. et al. Synthesis, characterization, and multielectron reduction chemistry of uranium supported by redox-active α-diimine ligands. Inorg. Chem. 50, 9838–9848 (2011).
Lu, E. & Liddle, S. T. Uranium-mediated oxidative addition and reductive elimination. Dalton Trans. 44, 12924–12941 (2015).
Acknowledgements
The authors thank H.S. La Pierre for discussions and M.E. Miehlich for assistance with EPR spectroscopy. D.P.H. acknowledges support from the Graduate School of Molecular Science (GSMS) of FAU Erlangen-Nürnberg. The Bundesministerium für Bildung und Forschung (BMBF, support codes 02NUK012C and 02NUK020C), the FAU Erlangen-Nürnberg and COST Action CM1006 are acknowledged for funding.
Author information
Authors and Affiliations
Contributions
D.P.H. and K.M. planned the research and prepared the manuscript. D.P.H. performed the experiments. F.W.H. conducted the XRD analyses and refined structures. L.M. performed theoretical calculations and analyses. All authors edited and reviewed the manuscript in the context of their contributions. K.M. supervised all aspects of the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 7414 kb)
Supplementary information
Crystallographic data for compound 2-K (CIF 1526 kb)
Supplementary information
Crystallographic data for compound 2 (CIF 8189 kb)
Supplementary information
Crystallographic data for compound 3 (CIF 3005 kb)
Supplementary information
Crystallographic data for compound 4 (CIF 7920 kb)
Rights and permissions
About this article
Cite this article
Halter, D., Heinemann, F., Maron, L. et al. The role of uranium–arene bonding in H2O reduction catalysis. Nature Chem 10, 259–267 (2018). https://doi.org/10.1038/nchem.2899
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2899
This article is cited by
-
Accessing five oxidation states of uranium in a retained ligand framework
Nature Communications (2023)
-
Terminal uranium(V)-nitride hydrogenations involving direct addition or Frustrated Lewis Pair mechanisms
Nature Communications (2020)
-
Metallacyclic actinide catalysts for dinitrogen conversion to ammonia and secondary amines
Nature Chemistry (2020)
-
δ and φ back-donation in AnIV metallacycles
Nature Communications (2020)
-
Transition-metal-bridged bimetallic clusters with multiple uranium–metal bonds
Nature Chemistry (2019)