Abstract
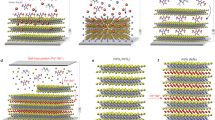
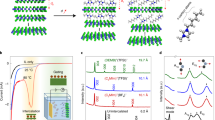
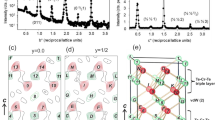
The controlled introduction of impurities into the crystal lattice of solid-state compounds is a cornerstone of materials science. Intercalation, the insertion of guest atoms, ions or molecules between the atomic layers of a host structure, can produce novel electronic, magnetic and optical properties in many materials. Here we describe an intercalation compound in which the host [Co6Te8(PnPr3)6][C60]3, formed from the binary assembly of atomically precise molecular clusters, is a superatomic analogue of traditional layered atomic compounds. We find that tetracyanoethylene (TCNE) can be inserted into the superstructure through a single-crystal-to-single-crystal transformation. Using electronic absorption spectroscopy, electrical transport measurements and electronic structure calculations, we demonstrate that the intercalation is driven by the exchange of charge between the host [Co6Te8(PnPr3)6][C60]3 and the intercalant TCNE. These results show that intercalation is a powerful approach to manipulate the material properties of superatomic crystals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schafhaeutl, C. Ueber die Verbindungen des Kohlenstoffes mit Silicium, Eisen und anderen Metallen, welche die verschiedenen Gallungen von Roheisen, Stahl und Schmiedeeisen bilden. J. Prakt. Chem. 21, 129–157 (1840).
Dresselhaus, M. S. & Dresselhaus, G. Advances in physics intercalation compounds of graphite. Adv. Phys. 51, 1–186 (1981).
Chan, C. K. et al. Fast, completely reversible Li insertion in vanadium pentoxide nanoribbons. Nano Lett. 7, 490–495 (2007).
Goodenough, J. B. & Park, K. S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Wan, C. et al. Flexible n-type thermoelectric materials by organic intercalation of layered transition metal dichalcogenide TiS2 . Nat. Mater. 14, 622–627 (2015).
Kovtyukhova, N. I. et al. Non-oxidative intercalation and exfoliation of graphite by Brønsted acids. Nat. Chem. 6, 957–963 (2014).
Nicolosi, V., Chhowalla, M., Kanatzidis, M. G., Strano, M. S. & Coleman, J. N. Liquid exfoliation of layered materials. Science 340, 1226419 (2013).
Fan, X. et al. Controlled exfoliation of MoS2 crystals into trilayer nanosheets. J. Am. Chem. Soc. 138, 5143–5149 (2016).
Li, X. et al. Direct visualization of the Jahn–Teller effect coupled to Na ordering in Na5/8MnO2 . Nat. Mater. 13, 586–592 (2014).
Yamanaka, S., Hotehama, K. & Kawaji, H. Superconductivity at 25.5 K in electron-doped layered hafnium nitride. Nature 392, 580–582 (1998).
Choi, B. et al. Van der Waals solids from self-assembled nanoscale building blocks. Nano Lett. 16, 1445–1449 (2016).
Turkiewicz, A. et al. Assembling hierarchical cluster solids with atomic precision. J. Am. Chem. Soc. 136, 15873–15876 (2014).
Tomalia, D. A. & Khanna, S. N. A systematic framework and nanoperiodic concept for unifying nanoscience: hard/soft nanoelements, superatoms, meta-atoms, new emerging properties, periodic property patterns, and predictive Mendeleev-like nanoperiodic tables. Chem. Rev. 116, 2705–2774 (2016).
Shores, M. P., Beauvais, L. G. & Long, J. R. Cluster-expanded Prussian blue analogues. J. Am. Chem. Soc. 121, 775–779 (1999).
Claridge, S. A. et al. Cluster-assembled materials. ACS Nano 3, 244–255 (2009).
Roy, X. et al. Nanoscale atoms in solid-state chemistry. Science 341, 157–160 (2013).
Baudron, S. A. et al. (EDT-TTF-CONH2)6[Re6Se8(CN)6], a metallic Kagome-type organic−inorganic hybrid compound: electronic instability, molecular motion, and charge localization. J. Am. Chem. Soc. 127, 11785–11797 (2005).
Lee, C.-H. et al. Ferromagnetic ordering in superatomic solids. J. Am. Chem. Soc. 136, 16926–16931 (2014).
Yoon, B. et al. Hydrogen-bonded structure and mechanical chiral response of a silver nanoparticle superlattice. Nat. Mater. 13, 807–811 (2014).
Ong, W.-L. et al. Orientational order controls crystalline and amorphous thermal transport in superatomic crystals. Nat. Mater. 16, 83–88 (2016).
Kovalenko, M. V. et al. Prospects of nanoscience with nanocrystals. ACS Nano 9, 1012–1057 (2015).
Shevchenko, E. V., Talapin, D. V., Kotov, N. A., O'Brien, S. & Murray, C. B. Structural diversity in binary nanoparticle superlattices. Nature 439, 55–59 (2006).
O'Brien, M. N., Jones, M. R., Lee, B. & Mirkin, C. A. Anisotropic nanoparticle complementarity in DNA-mediated co-crystallization. Nat. Mater. 14, 833–839 (2015).
Lee, J.-S., Kovalenko, M. V., Huang, J., Chung, D. S. & Talapin, D. V. Band-like transport, high electron mobility and high photoconductivity in all-inorganic nanocrystal arrays. Nat. Nanotech. 6, 348–352 (2011).
Xiao, W., Hu, C. & Ward, M. D. Guest exchange through single crystal–single crystal transformations in a flexible hydrogen-bonded framework. J. Am. Chem. Soc. 136, 14200–14206 (2014).
Batail, P. Tuning molecular solids. Science 341, 135–136 (2013).
Inokuma, Y., Arai, T. & Fujita, M. Networked molecular cages as crystalline sponges for fullerenes and other guests. Nat. Chem. 2, 780–783 (2010).
Bloch, E. D. et al. Metal insertion in a microporous metal–organic framework lined with 2,2′-bipyridine. J. Am. Chem. Soc. 132, 14382–14384 (2010).
Cargnello, M. et al. Substitutional doping in nanocrystal superlattices. Nature 524, 450–453 (2015).
Hebard, A. F. et al. Superconductivity at 18 K in potassium-doped C60 . Nature 350, 600–601 (1991).
Steigerwald, M. L., Siegrist, T. & Stuczynski, S. M. Octatelluridohexakis (triethylphosphine)hexacobalt and a connection between chevrel clusters and the NiAs structure. Inorg. Chem. 30, 2256–2257 (1991).
Anisimov, V. I., Zaanen, J. & Andersen, O. K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 44, 943–954 (1991).
Reed, C. A. & Bolskar, R. D. Discrete fulleride anions and fullerenium cations. Chem. Rev. 100, 1075–1120 (2000).
Stires, IV, J. C., McLaurin, E. J. & & Kubiak, C. P. Infrared spectroscopic determination of the degree of charge transfer in complexes of TCNE with methyl-substituted benzenes. Chem. Commun. 3532–3534 (2005).
Atallah, T. L., Gustafsson, M. V., Schmidt, E., Frisbie, C. D. & Zhu, X.-Y. Charge saturation and intrinsic doping in electrolyte-gated organic semiconductors. J. Phys. Chem. Lett. 6, 4840–4844 (2015).
Knupfer, M., Pichler, T., Golden, M. S. & Fink, J. The Physics of Fullerene-Based and Fullerene-Related Materials (ed. Andreoni, W.) 135–173 (Springer, 2000).
Andreoni, W. & Giannozzi, P. The Physics of Fullerene-Based and Fullerene-Related Materials (ed. Andreoni, W.) 291–329 (Springer, 2000).
Jehoulet, C., Obeng, Y. S., Kim, Y. T., Zhou, F. M. & Bard, A. J. Electrochemistry and Langmuir trough studies of C60 and C70 films. J. Am. Chem. Soc. 114, 4237–4247 (1992).
Connelly, N. G. & Geiger, W. E. Chemical redox potentials for organometallic chemistry. Chem. Rev. 96, 877–910 (1996).
Acknowledgements
We thank R. Hastie for her help in making the figures. Funding for this research was provided by the Center for Precision Assembly of Superstratic and Superatomic Solids, and the National Science Foundation (NSF) Materials Research Science and Engineering Centers (MRSEC) (Award no. DMR-1420634). The spectroscopy for this project was supported by the Air Force Office of Scientific Research (Award no. FA9550-14-1-0381). E.S.O'B., R.K. and J.C. are supported by the MRSEC. G.A.E. acknowledges support by the Semiconductor Research Cooperation–Nanoelectronics Research Initiative Hans J. Coufal Fellowship and the Columbia Optics and Quantum Electronics NSF Integrative Graduate Education and Research Traineeship (DGE-1069240). X-ray diffraction measurements were performed in the Shared Materials Characterization Laboratory at Columbia University. Use of the Shared Materials Characterization Laboratory was made possible by funding from Columbia University. We thank C. Nuckolls, M. Steigerwald, L. Brus and C. Dean for the use of their instruments and for useful discussions.
Author information
Authors and Affiliations
Contributions
E.S.O'B. synthesized the materials and, together with D.W.P., conducted the SCXRD characterization. M.T.T. and T.L.A. conducted the optical measurements. J.C., A.J.M. and D.R.R. formulated and performed the theoretical calculations. R.L.K., G.A.E. and A.M. fabricated the electrical devices and performed the electrical transport and Seebeck coefficient measurements. M.V.P., N.P. and A.C.C. measured the Raman spectra. E.S.O'B. wrote the manuscript with the help of M.T.T., and J.C., X.R., I.K., A.C.C., A.J.M., D.R.R. and X.Z. edited the manuscript. All the authors discussed the data and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1553 kb)
Supplementary information
Crystallographic data for compound [Co6Te8(PnPr3)6][C60]3[Toluene]6 (CIF 6811 kb)
Supplementary information
Crystallographic data for desolvated compound [Co6Te8(PnPr3)6][C60]3 (CIF 2004 kb)
Supplementary information
Crystallographic data for compound [Co6Te8(PnPr3)6][C60]3[TCNE]2 (CIF 1762 kb)
Rights and permissions
About this article
Cite this article
O'Brien, E., Trinh, M., Kann, R. et al. Single-crystal-to-single-crystal intercalation of a low-bandgap superatomic crystal. Nature Chem 9, 1170–1174 (2017). https://doi.org/10.1038/nchem.2844
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2844
This article is cited by
-
A family of ionic supersalts with covalent-like directionality and unconventional multiferroicity
Nature Communications (2021)
-
Superatomic solid solutions
Nature Chemistry (2021)
-
Giant single-crystal-to-single-crystal transformations associated with chiral interconversion induced by elimination of chelating ligands
Nature Communications (2021)
-
Engineering covalently bonded 2D layered materials by self-intercalation
Nature (2020)
-
Superatoms in materials science
Nature Reviews Materials (2020)