Abstract
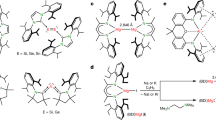
Mononuclear gold(II) complexes are very rare labile species. Transient gold(II) species have been suggested in homogeneous catalysis and in medical applications, but their geometric and electronic structures have remained essentially unexplored: even fundamental data, such as the ionic radius of gold(II), are unknown. Now, an unprecedentedly stable neutral gold(II) complex of a porphyrin derivative has been isolated, and its structural and spectroscopic features determined. The gold atom adopts a 2+2 coordination mode in between those of gold(III) (four-coordinate square planar) and gold(I) (two-coordinate linear), owing to a second-order Jahn–Teller distortion enabled by the relativistically lowered 6s orbital of gold. The reactivity of this gold(II) complex towards dioxygen, nitrosobenzene and acids is discussed. This study provides insight on the ionic radius of gold(II), and allows it to be placed within the homologous series of nd9 Cu/Ag/Au divalent ions and the 5d8/9/10 Pt/Au/Hg ‘relativistic’ triad in the periodic table.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Fürstner, A. Gold and platinum catalysis—a convenient tool for generating molecular complexity. Chem. Soc. Rev. 38, 3208–3221 (2009).
Hashmi, A. S. K. Dual gold catalysis. Acc. Chem. Res. 47, 864–876 (2014).
Dorel, R. & Echavarren, A. M. Gold(I)-catalyzed activation of alkynes for the construction of molecular complexity. Chem. Rev. 115, 9028–9072 (2015).
Hashmi, A. K. S. Homogeneous gold catalysis beyond assumptions and proposals—characterized intermediates. Angew. Chem. Int. Ed. 49, 5232–5241 (2010).
Liu, L. P. & Hammond, G. B. Recent advances in the isolation and reactivity of organogold complexes. Chem. Soc. Rev. 41, 3129–3139 (2012).
Echavarren, A. M. & Obradors, C. Intriguing mechanistic labyrinths in gold(I) catalysis. Chem. Commun. 50, 16–28 (2014).
Joost, M., Amgoune, A. & Bourissou, D. Reactivity of gold complexes towards elementary organometallic reactions. Angew. Chem. Int. Ed. 54, 15022–15045 (2015).
Sahoo, B., Hopkinson, M. N. & Glorius, F. Combining gold and photoredox catalysis: visible light-mediated oxy- and aminoarylation of alkenes. J. Am. Chem. Soc. 135, 5505–5508 (2013).
Winston, M. S., Wolf, W. J. & Toste, F. D. Photoinitiated oxidative addition of CF3I to gold(I) and facile aryl–CF3 reductive elimination. J. Am. Chem. Soc. 136, 7777–7782 (2014).
Shu, X., Zhang, M., He, Y., Frei, H. & Toste, F. D. Dual visible light photoredox and gold-catalyzed arylative ring expansion. J. Am. Chem. Soc. 136, 5844–5847 (2014).
Tlahuext-Aca, A., Hopkinson, M. N., Sahooab, B. & Glorius, F. Dual gold/photoredox-catalyzed C(sp)–H arylation of terminal alkynes with diazonium salts. Chem. Sci. 7, 89–93 (2016).
Hopkinson, M. N., Tlahuext-Aca, A. & Glorius, F. Merging visible light photoredox and gold catalysis. Acc. Chem. Res. 49, 2261–2272 (2016).
Huang, L., Rudolph, M., Rominger, F. & Hashmi, A. S. K. Photosensitizer-free visible-light-mediated gold-catalyzed 1,2-difunctionalization of alkynes. Angew. Chem. Int. Ed. 55, 4808–4813 (2016).
Kim, S., Rojas-Martin, J. & Toste, D. F. Visible light-mediated gold-catalysed carbon(sp2)–carbon(sp) cross-coupling. Chem. Sci. 7, 85–88 (2016).
Gimeno, M. C. & Laguna, A. in Comprehensive Coordination Chemistry II Vol. 6 (eds McCleverty, J. A. & Meyer, T. J.) 999–1145 (Elsevier, 2005).
Mohamed, A. A., Abdou, H. E. & Fackler, J. P. Jr. Coordination chemistry of gold(II) with amidinate, thiolate and ylide ligands. Coord. Chem. Rev. 254, 1253–1259 (2010).
Elder, S. H., Lucier, G. M., Hollander, F. J. & Bartlett, N. Synthesis of Au(II) fluoro complexes and their structural and magnetic properties. J. Am. Chem. Soc. 119, 1020–1026 (1997).
Blake, A. J. et al. Bis(1,4,7-trithiacyclononane)gold dication: a paramagnetic, mononuclear AuII complex. Angew. Chem. Int. Ed. 29, 197–198 (1990).
Seidel, S. & Seppelt, K. Xenon as a complex ligand: the tetra xenono gold(II) cation in AuXe42+(Sb2F11–)2 . Science 290, 117–118 (2000).
Drews, T., Seidel, S. & Seppelt, K. Gold–xenon complexes. Angew. Chem. Int. Ed. 41, 454–456 (2002).
MacCragh, A. & Koski, W. S. The phthalocyanine of gold. J. Am. Chem. Soc. 87, 2496–2497 (1965).
Wong, E. W. Y. et al. Gold(II) phthalocyanine revisited: synthesis and spectroscopic properties of gold(III) phthalocyanine and an unprecedented ring-contracted phthalocyanine analogue. Chem. Eur. J. 18, 12404–12410 (2012).
Brun, A. M., Harriman, A., Heitz, V. & Sauvage, J. P. Charge transfer across oblique bisporphyrins: two-center photoactive molecules. J. Am. Chem. Soc. 113, 8657–8663 (1991).
Fukuzumi, S. et al. Metal-centered photoinduced electron transfer reduction of a gold(III) porphyrin cation linked with a zinc porphyrin to produce a long-lived charge-separated state in nonpolar solvents. J. Am. Chem. Soc. 125, 14984–14985 (2003).
Fortage, J. et al. Single-step electron transfer on the nanometer scale: ultra-fast charge shift in strongly coupled zinc porphyrin–gold porphyrin dyads. Chem. Eur. J. 14, 3467–3480 (2008).
Kadish, K. M. et al. Evidence that gold(III) porphyrins are not electrochemically inert: facile generation of gold(II) 5,10,15,20-tetrakis(3,5-di-tert-butylphenyl)porphyrin. Chem. Commun. 356–357 (2002).
Ou, Z. et al. Substituent effects on the site of electron transfer during the first reduction for gold(III) porphyrins. Inorg. Chem. 43, 2078–2086 (2004).
Preiß, S., Melomedov, J., Wünsche von Leupoldt, A. & Heinze, K. Gold(III) tetraarylporphyrin amino acid derivatives: ligand or metal centred redox chemistry? Chem. Sci. 7, 596–610 (2016).
Che, C.-M. et al. Gold(III) porphyrins as a new class of anticancer drugs: cytotoxicity, DNA binding and induction of apoptosis in human cervix epithelioid cancer cells. Chem. Commun. 1718–1719 (2003).
Wang, Y., He, Q. Y., Sun, R. W., Che, C. M. & Chiu, J. F. Gold(III) porphyrin 1a induced apoptosis by mitochondrial death pathways related to reactive oxygen species. Cancer Res. 65, 11553–11564 (2005).
Lum, C. T., Sun, R. W.-Y., Zou, T. & Che, C.-M. Gold(III) complexes inhibit growth of cisplatin-resistant ovarian cancer in association with upregulation of proapoptotic PMS2 gene. Chem. Sci. 5, 1579–1584 (2014).
Hu, D. et al. Anticancer gold(III) porphyrins target mitochondrial chaperone Hsp60. Angew. Chem. Int. Ed. 55, 1387–1391 (2016).
Manoharan, P. T. & Rogers, M. T. in Electron Spin Resonance of Metal Complexes (ed. Yen, T. F.) 143–173 (Plenum, 1969).
Hazell, A. Structure of (5,10,15,20-tetraphenyl-21H,23H-porphinato)platinum(II), C44H28N4Pt. Acta Cryst. C 40, 751–753 (1984).
Silvers, S. J. & Tulinsky, A. The crystal and molecular structure of triclinic tetraphenylporphyrin. J. Am. Chem. Soc. 89, 3331–3337 (1967).
Scheidt, W. R. et al. Crystal and molecular structure of the silver(II) and zinc(II) derivatives of meso-tetraphenylporphyrin. An exploration of crystal-packing effects on bond distance. Inorg. Chem. 25, 795–799 (1986).
Plaza, L. A. & Chojnacki, J. Influence of chloroform on crystalline products yielded in reactions of 5,10,15,20-tetraphenylporphyrin with HCl and copper(II) salts. Acta Cryst. C 86, m24–m28 (2012).
Bayler, A., Schier, A., Bowmaker, G. A. & Schmidbaur, H. Gold is smaller than silver. Crystal structures of [bis(trimesitylphosphine)gold(I)] and [bis(trimesitylphosphine)silver(I)] tetrafluoroborate. J. Am. Chem. Soc. 118, 7006–7007 (1996).
Pearson, R. G. Concerning Jahn–Teller effects. Proc. Natl Acad. Sci. USA 72, 2104–2106 (1975).
Leyva-Pérez, A. & Corma, A. Similarities and differences between the ‘relativistic’ triad gold, platinum, and mercury in catalysis. Angew. Chem. Int. Ed. 51, 614–635 (2012).
Pyykkö, P. Theoretical chemistry of gold. III. Chem. Soc. Rev. 37, 1967–1997 (2008).
Bojan, R. V. et al. Double Jahn–Teller distortion in AuGe complexes leading to a dual blue–orange emission. ChemPlusChem 81, 176–186 (2016).
Wang, M. C. et al. Mercury complexes of meso-tetra-(p-cyanophenyl)porphyrin and N-methylporphyrin: meso-tetra(p-cyanophenyl)porphyrinatomercury(II) and chloro(N-methyl-meso-tetraphenylporphyrinato) mercury(II). Inorg. Chem. 40, 6064–6068 (2001).
Antipas, A., Dolphin, D., Gouterman, M. & Johnson, E. C. Porphyrins. 38. Redox potentials, charge transfer transitions, and emission of copper, silver, and gold complexes. J. Am. Chem. Soc. 100, 7705–7709 (1978).
Koppenol, W. H., Stanbury, D. M. & Bounds, P. L. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic. Biol. Med. 49, 317–322 (2010).
Roşca, D.-A., Wright, J. A., Hughes, D. L. & Bochmann, M. Gold peroxide complexes and the conversion of hydroperoxides into gold hydrides by successive oxygen-transfer reactions. Nat. Commun. 4, 2167 (2013).
Dann, T. et al. Electrochemistry of AuII and AuIII pincer complexes: determination of the AuII–AuII bond energy. Chem. Commun. 49, 10169–10171 (2013).
Neidlinger, A., Kienz, T. & Heinze, K. Spin trapping of carbon-centered ferrocenyl radicals with nitrosobenzene. Organometallics 34, 5310–5320 (2015).
Núñez-Vergara, L. J. et al. Nitrosobenzene: electrochemical, UV–visible and EPR spectroscopic studies on the nitrosobenzene free radical generation and its interaction with glutathione. Electrochim. Acta 45, 3555–3561 (2000).
Zhu, X.-Q. et al. Hydride, hydrogen atom, proton, and electron transfer driving forces of various five-membered heterocyclic organic hydrides and their reaction intermediates in acetonitrile. J. Am. Chem. Soc. 130, 2501–2516 (2008).
Acknowledgements
Parts of this research were conducted using the supercomputer Mogon and advisory services offered by Johannes Gutenberg University Mainz (www.hpc.uni-mainz.de), which is a member of the AHRP and the Gauss Alliance. We thank P. Auerbach and M. Mondeshki for collecting the LIFDI mass spectra and assistance with the paramagnetic NMR spectra and R. Jung-Pothmann for collection of the diffraction data. This work was financially supported by the Deutsche Forschungsgemeinschaft (GSC 266, Materials Science in Mainz, scholarship for S.O.). PETRA III is acknowledged for the provision of beamtime at beamline P64. This article is dedicated to G. Huttner on the occasion of his 80th birthday.
Author information
Authors and Affiliations
Contributions
S.P. synthesized [Au(TPP)][PF6] and Au(TPP), measured the X-band EPR, NMR, infrared, UV–Vis and mass spectra and studied the reactivity, S.O. made the quantum chemical calculations, C.F. performed the single-crystal XRD analysis, D.H. and H.H.H. measured and interpreted the Q-band EPR spectra, P.M. and M.B. measured and interpreted the X-ray absorption spectra and L.C. measured the magnetic susceptibility data. K.H. conceived and designed the experiments and wrote the paper. All of the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2876 kb)
Supplementary information
Crystallographic data for compound Au(TPP) (CIF 294 kb)
Supplementary information
Structure factors file for compound Au(TPP) (FCF 210 kb)
Rights and permissions
About this article
Cite this article
Preiß, S., Förster, C., Otto, S. et al. Structure and reactivity of a mononuclear gold(II) complex. Nature Chem 9, 1249–1255 (2017). https://doi.org/10.1038/nchem.2836
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2836
This article is cited by
-
Stabilizing Au2+ in a mixed-valence 3D halide perovskite
Nature Chemistry (2023)
-
Gold carbene complexes and beyond: new avenues in gold(I)-carbon coordination chemistry
Gold Bulletin (2022)
-
Quantitative Selection of the Axially Chiral Conformation in a Flexible Dinuclear Gold(I) di(N-Heterocyclic Carbene) Complex via Chlorine Oxidative Addition
Journal of Chemical Crystallography (2021)
-
Electrochemical oxidative aminocarbonylation of terminal alkynes
Nature Catalysis (2020)