Abstract
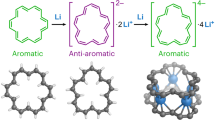
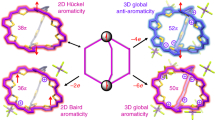
Classic formulations of aromaticity have long been associated with topologically planar conjugated macrocyclic systems. The theoretical possibility of so-called bicycloaromaticity was noted early on. However, it has yet to be demonstrated by experiment in a simple synthetic organic molecule. Conjugated organic systems are attractive for studying the effect of structure on electronic features. This is because, in principle, they can be modified readily through dedicated synthesis. As such, they can provide useful frameworks for testing by experiment with fundamental insights provided by theory. Here we detail the synthesis and characterization of two purely organic non-planar dithienothiophene-bridged [34]octaphyrins that permit access to two different aromatic forms as a function of the oxidation state. In their neutral forms, these congeneric systems contain competing 26 and 34 π-electronic circuits. When subject to two-electron oxidation, electronically mixed [4n+1]/[4n+1] triplet biradical species in the ground state are obtained that display global aromaticity in accord with Baird's rule.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hofmann, A. W. On insolinic acid. Proc. R. Soc. Lond. 8, 1–3 (1856).
Rocke, A. J. It began with a daydream: The 150th anniversary of the Kekulé benzene structure. Angew. Chem. Int. Ed. 54, 46–50 (2015).
Hückel, E. Quantentheoretische beiträge zum benzolproblem. Z. Phys. 70, 204–286 (1931).
Breslow, R. Antiaromaticity. Acc. Chem. Res. 6, 393–398 (1973).
van Tamelen, E. E. & Burkoth, T. L. Cyclodecapentaene. J. Am. Chem. Soc. 89, 151–152 (1967).
Wu, J. I.-C., Mo, Y., Evangelista, F. A. & Schleyer, P. v. R. Is cyclobutadiene really highly destabilized by antiaromaticity? Chem. Commun. 48, 8437–8439 (2012).
Krieger, C., Diederich, F., Schweitzer, D. & Staab, H. A. Molecular structure and spectroscopic properties of kekulene. Angew. Chem. Int. Ed. 18, 699–701 (1979).
Aihara, J. Is superaromaticity a fact or an artifact? The kekulene problem. J. Am. Chem. Soc. 114, 865–868 (1992).
Breslow, R. & Groves, J. T. Cyclopropenyl cation. Synthesis and characterization. J. Am. Chem. Soc. 92, 984–987 (1970).
Heilbronner, E. Hückel molecular orbitals of Möbius-type conformations of annulenes. Tetrahedron Lett. 5, 1923–1928 (1964).
Ajami, D., Oeckler, O., Simon, A. & Herges, R. Synthesis of a Möbius aromatic hydrocarbon. Nature 426, 819–821 (2003).
Baird, N. C. Quantum organic photochemistry. II. Resonance and aromaticity in the lowest 3ππ* state of cyclic hydrocarbons. J. Am. Chem. Soc. 94, 4941–4948 (1972).
Shin, J.-Y. et al. Aromaticity and photophysical properties of various topology-controlled expanded porphyrins. Chem. Soc. Rev. 39, 2751–2767 (2010).
Zhang, Z. et al. Cyclo[m]pyridine[n]pyrroles: hybrid macrocycles that display expanded π-conjugation upon protonation. J. Am. Chem. Soc. 134, 4076–4079 (2012).
Ishida, M. et al. Protonation-coupled redox reactions in planar antiaromatic meso-pentafluorophenyl-substituted o-phenylene-bridged annulated rosarins. Nat. Chem. 5, 15–20 (2013).
Simkowa, I., Latos-Grazynski, L. & Stepien, M. π Conjugation transmitted across a d-electron metallocene in ferrocenothiaporphyrin macrocycles. Angew. Chem. Int. Ed. 49, 7665–7669 (2010).
Rosenberg, M., Dahlstrand, C., Kilsa, K. & Ottoson, H. Excited state aromaticity and antiaromaticity: opportunities for photophysical and photochemical rationalizations. Chem. Rev. 114, 5379–5425 (2014).
Sung, Y. M. et al. Reversal of Hückel (anti)aromaticity in the lowest triplet states of hexaphyrins and spectroscopic evidence for Baird's rule. Nat. Chem. 7, 418–422 (2015).
Sung, Y. M. et al. Switching between aromatic and antiaromatic 1,3-phenylene-strapped [26]- and [28]hexaphyrins upon passage to the singlet excited state. J. Am. Chem. Soc. 137, 11856–11859 (2015).
Oh, J. et al. Aromaticity reversal in the lowest excited triplet state of archetypical Möbius heteroannulenic systems. Angew. Chem. Int. Ed. 55, 6487–6491 (2016).
Goldstein, M. J. & Hoffmann, R. Symmetry, topology, and aromaticity. J. Am. Chem. Soc. 93, 6193–6204 (1967).
Goldstein, M. J. Bicycloaromaticity. 4m + 2, 4n rule. J. Am. Chem. Soc. 89, 6357–6359 (1967).
Karthik, G. et al. Core-modified meso-aryl hexaphyrins with an internal thiophene bridge: structure, aromaticity, and photodynamics. Chem. Eur. J. 19, 1886–1890 (2013).
Bucher, C., Zimmerman, R. S., Lynch, V. & Sessler, J. L. First cryptand-like calixpyrrole: synthesis, X-ray structure, and anion binding properties of a bicyclic[3,3,3]nonapyrrole. J. Am. Chem. Soc. 123, 9716–9717 (2001).
Bucher, C., Zimmerman, R. S., Lynch, V. & Sessler, J. L. Synthesis and X-ray structure of a three-dimensional calixphyrin. Chem. Commun. 10, 1646–1647 (2003).
Karthik, G. et al. Phenylene-bridged core-modified planar aromatic octaphyrin: aromaticity, photophysical and anion receptor properties. Chem. Asian J. 10, 1447–1453 (2016).
Chandrashekar, T. K., Prabhuraja, V., Gokulnath, S., Sabarinathan, R. & Srinivasan, A. Fused core-modified meso-aryl expanded porphyrins. Chem. Commun. 46, 5915–5917 (2010).
Gopalakrishna, T. Y., Reddy, J. S. & Anand, V. G. An amphoteric switch to aromatic and antiaromatic states of a neutral air-stable 25π radical. Angew. Chem. Int. Ed. 53, 10984–10987 (2014).
Yoshida, T., Zhou, W., Furuyama, T., Leznoff, D. B. & Kobayashi, N. An extremely air-stable 19π porphyrinoid. J. Am. Chem. Soc. 137, 9258–9261 (2015).
Fukuzumi, S. et al. Formation of ground state triplet diradicals from annulated rosarin derivatives by triprotonation. J. Am. Chem. Soc. 137, 9780–9783 (2015).
Bleaney, B. & Bowers, K. D. Anomalous paramagnetism of copper acetate. Proc. R. Soc. Lond. A 214, 451–465 (1952).
Acknowledgements
The research at Yonsei University was supported by the Samsung Science and Technology Foundation (SSTF-BA1402-10). The quantum calculations were performed using the supercomputing resources of the Korea Institute of Science and Technology Information. T.K.C. thanks the Department of Science and Technology, New Delhi, India, for a J. C. Bose Fellowship. The work in Austin was supported by the National Science Foundation (grant CHE-1402004) and the Robert A. Welch Foundation. The work at Sookmyung Women's University was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (NRF-2016R1C1B2014895). This work was also supported by the Japanese Society for the Promotion of Science (grant no. 16H02268 to S.F.).
Author information
Authors and Affiliations
Contributions
D.K. and J.L.S. conceived and designed this work. W.-Y.C., A.G., R.A., J.S.P. and T.K.C. designed and synthesized the materials. Z.Z., X.-S.K. and V.M.L. obtained the single-crystal X-ray diffraction structures. J.J., W.-Y.C. and S.F. performed and analysed the EPR measurements. W.-Y.C. and T.K. performed the computational calculations. W.-Y.C., T.K., W.K. and S.L. performed the spectroscopic measurements. D.K., J.L.S. and W.-Y.C. co-wrote the paper. D.K. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2707 kb)
Rights and permissions
About this article
Cite this article
Cha, WY., Kim, T., Ghosh, A. et al. Bicyclic Baird-type aromaticity. Nature Chem 9, 1243–1248 (2017). https://doi.org/10.1038/nchem.2834
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2834
This article is cited by
-
Isolation of a triplet benzene dianion
Nature Chemistry (2021)
-
Global aromaticity at the nanoscale
Nature Chemistry (2020)
-
3D global aromaticity in a fully conjugated diradicaloid cage at different oxidation states
Nature Chemistry (2020)
-
Two-electron transfer stabilized by excited-state aromatization
Nature Communications (2019)