Abstract
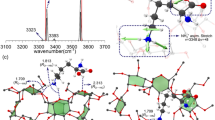
The amino acid serine is known to form a very stable octamer that has properties that set it apart from serine complexes of different sizes or from complexes composed of other amino acids. For example, both singly protonated serine octamers and anionic octamers complexed with two halogen ions strongly prefer homochirality, even when assembled from racemic D,L mixtures. Consequently, the structures of these complexes are of great interest, but no acceptable candidates have so far been identified. Here, we investigate anionic serine octamers coordinated with two chloride ions using a novel technique coupling ion mobility spectrometry–mass spectrometry with infrared spectroscopy, in combination with theoretical calculations. The results allow the identification of a unique structure for (Ser8Cl2)2− that is highly symmetric, very stable and homochiral and whose calculated properties match those observed in experiments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Echt, O., Sattler, K. & Recknagel, E. Magic numbers for sphere packings—experimental verification in free xenon clusters. Phys. Rev. Lett. 47, 1121–1124 (1981).
Kroto, H. W., Heath, J. R., O'Brian, S. C., Curl, R. F. & Smalley, R. E. C60: buckminsterfullerene. Nature 318, 162–163 (1985).
Guo, B. C., Wei, S., Purnell, J., Buzza, S. & Castleman, A. W. Metallo-carbohedrenes [M8C12+ (M = V, Zr, Hf, and Ti)]—a class of stable molecular cluster ions. Science 256, 515–516 (1992).
Li, J., Li, X., Zhai, H. J. & Wang, L. S. Au20: a tetrahedral cluster. Science 299, 864–867 (2003).
Gruene, P. et al. Structures of neutral Au7, Au19, and Au20 clusters in the gas phase. Science 321, 674–676 (2008).
Miyazaki, M., Fujii, A., Ebata, T. & Mikami, N. Infrared spectroscopic evidence for protonated water clusters forming nanoscale cages. Science 304, 1134–1137 (2004).
Shin, J. W. et al. Infrared signature of structures associated with the H+(H2O)n (n = 6 to 27) clusters. Science 304, 1137–1140 (2004).
Cooks, R. G., Zhang, D., Koch, K. J., Gozzo, F. C. & Eberlin, M. N. Chiroselective self-directed octamerization of serine: implications for homochirogenesis. Anal. Chem. 73, 3646–3655 (2001).
Nemes, P., Schlosser, G. & Vékey, K. Amino acid cluster formation studied by electrospray ionization mass spectrometry. J. Mass Spectrom. 40, 43–49 (2005).
Kellermeier, M. et al. Amino acids form prenucleation clusters: ESI-MS as a fast detection method in comparison to analytical ultracentrifugation. Faraday Discuss. 159, 23–45 (2012).
Feketeová, L. et al. Fragmentation of the tryptophan cluster [Trp9–2H]2− induced by different activation methods. Rapid Commun. Mass Spectrom. 24, 3255–3260 (2010).
Counterman, A. E. & Clemmer, D. E. Anhydrous polyproline helices and globules. J. Phys. Chem. B 108, 4885–4898 (2004).
Julian, R. R., Hodyss, R. & Beauchamp, J. L. Salt bridge stabilization of charged zwitterionic arginine aggregates in the gas phase. J. Am. Chem. Soc. 123, 3577–3583 (2001).
Do, T. D. et al. Amino acid metaclusters: implications of growth trends on peptide self-assembly and structure. Anal. Chem. 88, 868–876 (2016).
Nanita, S. C., Takats, Z., Cooks, R. G., Myung, S. & Clemmer, D. E. Chiral enrichment of serine via formation, dissociation, and soft-landing of octameric cluster ions. J. Am. Soc. Mass Spectrom. 15, 1360–1365 (2004).
Nanita, S. C. & Cooks, R. G. Serine octamers: cluster formation, reactions, and implications for biomolecule homochirality. Angew. Chem. Int. Ed. 45, 554–569 (2006).
Koch, K. J., Gozzo, F. C., Zhang, D., Eberlin, M. N. & Cooks, R. G. Serine octamer metaclusters: formation, structure elucidation and implications for homochiral polymerization. Chem. Commun. 1854–1855 (2001).
Nanita, S. C. & Cooks, R. G. Negatively-charged halide adducts of homochiral serine octamers. J. Phys. Chem. B 109, 4748–4753 (2005).
Schalley, C. A. & Weis, P. Unusually stable magic number clusters of serine with a surprising preference for homochirality. Int. J. Mass Spectrom. 221, 9–19 (2002).
Julian, R. R., Hodyss, R., Kinnear, B., Jarrold, M. F. & Beauchamp, J. L. Nanocrystalline aggregation of serine detected by electrospray ionization mass spectrometry: origin of the stable homochiral gas-phase serine octamer. J. Phys. Chem. B 106, 1219–1228 (2002).
Counterman, A. E. & Clemmer, D. E. Magic number clusters of serine in the gas phase. J. Phys. Chem. B 105, 8092–8096 (2001).
Clemmer, D. E. & Jarrold, M. F. Ion mobility measurements and their applications to clusters and biomolecules. J. Mass Spectrom. 32, 577–592 (1997).
Wyttenbach, T. & Bowers, M. T. Gas-phase conformations: the ion mobility/ion chromatography method. Mod. Mass Spectrom. 225, 207–232 (2003).
Kanu, A. B. et al. Ion mobility-mass spectrometry. J. Mass Spectrom. 43, 1–22 (2008).
Warnke, S. et al. Protomers of benzocaine solvent and permittivity dependence. J. Am. Chem. Soc. 137, 4236–4242 (2015).
Masson, A. et al. Infrared spectroscopy of mobility-selected H+-Gly-Pro-Gly-Gly (GPGG). J. Am. Soc. Mass Spectrom. 26, 1444–1454 (2015).
Seo, J. et al. The impact of environment and resonance effects on the site of protonation of aminobenzoic acid derivatives. Phys. Chem. Chem. Phys. 18, 25474–25482 (2016).
Voronina, L. et al. Conformations of prolyl–peptide bonds in the bradykinin 1–5 fragment in solution and in the gas phase. J. Am. Chem. Soc. 138, 9224–9233 (2016).
Seo, J. et al. An infrared spectroscopy approach to follow β-sheet formation in peptide amyloid assemblies. Nat. Chem. 9, 39–44 (2017).
Kong, X. et al. Progressive stabilization of zwitterionic structures in [H(Ser)2–8]+ studied by infrared photodissociation spectroscopy. Angew. Chem. Int. Ed. 45, 4130–4134 (2006).
Kong, X. et al. Numerous isomers of serine octamer ions characterized by infrared photodissociation spectroscopy. ChemPhysChem 10, 2603–2606 (2009).
Sunahori, F. X., Yang, G., Kitova, E. N., Klassen, J. S. & Xu, Y. Chirality recognition of the protonated serine dimer and octamer by infrared multiphoton dissociation spectroscopy. Phys. Chem. Chem. Phys. 15, 1873–1886 (2013).
Takats, Z., Nanita, S. C., Schlosser, G., Vekey, K. & Cooks, R. G. Atmospheric pressure gas-phase H/D exchange of serine octamers. Anal. Chem. 75, 6147–6154 (2003).
Linder, R., Seefeld, K., Vavra, A. & Kleinermanns, K. Gas phase infrared spectra of nonaromatic amino acids. Chem. Phys. Lett. 453, 1–6 (2008).
Antony, J., von Helden, G., Meijer, G. & Schmidt, B. Anharmonic midinfrared vibrational spectra of benzoic acid monomer and dimer. J. Chem. Phys. 123, 014305 (2005).
Kapota, C., Lemaire, J., Maitre, P. & Ohanessian, G. Vibrational signature of charge solvation vs salt bridge isomers of sodiated amino acids in the gas phase. J. Am. Chem. Soc. 126, 1836–1842 (2004).
Oomens, J., Steill, J. D. & Redlich, B. Gas-phase IR spectroscopy of deprotonated amino acids. J. Am. Chem. Soc. 131, 4310–4319 (2009).
Blom, M. N. et al. Stepwise solvation of an amino acid: the appearance of zwitterionic structures. J. Phys. Chem. A 111, 7309–7316 (2007).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Vandenbussche, S., Vandenbussche, G., Reisse, J. & Bartik, K. Do serine octamers exist in solution? relevance of this question in the context of the origin of homochirality on earth. Eur. J. Org. Chem. 2006, 3069–3073 (2006).
Kemper, P. R., Dupuis, N. F. & Bowers, M. T. A new, higher resolution, ion mobility mass spectrometer. Int. J. Mass Spectrom. 287, 46–57 (2009).
Warnke, S., Baldauf, C., Bowers, M. T., Pagel, K. & von Helden, G. Photodissociation of conformer-selected ubiquitin ions reveals site-specific cis/trans isomerization of proline peptide bonds. J. Am. Chem. Soc. 136, 10308–10314 (2014).
Warnke, S., von Helden, G. & Pagel, K. Analyzing the higher order structure of proteins with conformer-selective ultraviolet photodissociation. Proteomics 15, 2804–2812 (2015).
Mason, E. A. & McDaniel, E. W. Transport Properties of Ions in Gases (Wiley, 2005).
Schöllkopf, W. et al. in Advances in X-Ray Free-Electron Lasers Instrumentation III Vol. 9512 (ed. Biedron, S. G.) (SPIE, 2015).
MacroModel (Schrödinger, 2016); https://www.schrodinger.com/macromodel.
Gaussian 09, Revision D.01 (Gaussian, 2009); http://gaussian.com/glossary/g09/.
Kesharwani, M. K., Brauer, B. & Martin, J. M. L. Frequency and zero-point vibrational energy scale factors for double-hybrid density functionals (and other selected methods): can anharmonic force fields be avoided? J. Phys. Chem. A 119, 1701–1714 (2015).
Mesleh, M. F., Hunter, J. M., Shvartsburg, A. A., Schatz, G. C. & Jarrold, M. F. Structural information from ion mobility measurements: effects of the long-range potential. J. Phys. Chem. 100, 16082–16086 (1996).
Acknowledgements
The authors acknowledge the expert assistance of the FHI free electron laser facility staff, in particular S. Gewinner and W. Schöllkopf. M.T.B. acknowledges the Alexander von Humboldt Foundation and the National Science Foundation (USA) for support under grants CHE-1301032 and CHE-1565941.
Author information
Authors and Affiliations
Contributions
J.S., S.W., K.P., M.T.B. and G.v.H. conceived and designed the experiments. J.S. and S.W. performed the experiments. J.S. analysed the data and suggested the structure of the serine octamer with two chlorides. All authors co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1584 kb)
Rights and permissions
About this article
Cite this article
Seo, J., Warnke, S., Pagel, K. et al. Infrared spectrum and structure of the homochiral serine octamer–dichloride complex. Nature Chem 9, 1263–1268 (2017). https://doi.org/10.1038/nchem.2821
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2821
This article is cited by
-
The emerging interface of mass spectrometry with materials
NPG Asia Materials (2019)
-
Chiral Differentiation of Non-Covalent Diastereomers Based on Multichannel Dissociation Induced by 213-nm Ultraviolet Photodissociation
Journal of the American Society for Mass Spectrometry (2019)
-
Fundamental Studies of New Ionization Technologies and Insights from IMS-MS
Journal of the American Society for Mass Spectrometry (2019)
-
Deciphering key intermediates in the transformation of carbon dioxide into heterocyclic products
Nature Catalysis (2018)