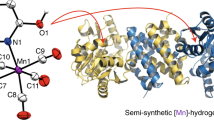
Abstract
Mono-iron hydrogenase was the third type of hydrogenase discovered. Its Lewis acidic iron(II) centre promotes the heterolytic cleavage of the H–H bond and this non-redox H2 activation distinguishes it from the well-studied dinuclear [FeFe] and [NiFe] hydrogenases. Cleavage of the H–H bond is followed by hydride transfer to the enzyme's organic substrate, H4MPT+, which serves as a CO2 ‘carrier’ in methanogenic pathways. Here we report a scaffold-based synthetic approach by which to model mono-iron hydrogenase using an anthracene framework, which supports a biomimetic fac-C,N,S coordination motif to an iron(II) centre. This arrangement includes the biomimetic and organometallic Fe–C σ bond, which enables bidirectional activity reminiscent of the native enzyme: the complex activates H2 under mild conditions, and catalyses C–H hydride abstraction plus H2 generation from a model substrate. Notably, neither H2 activation nor C–H hydride abstraction was observed in the analogous complex with a pincer-type mer-C,N,S ligation, emphasizing the importance of the fac-C,N,S-iron(II) motif in promoting enzyme-like reactivity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zhou, J., Hu, Z., Münck, E. & Holm, R. H. The cuboidal Fe3S4 cluster: synthesis, stability, and geometric and electronic structures in a non-protein environment. J. Am. Chem. Soc. 118, 1966–1980 (1996).
Kanady, J. S., Tsui, E. Y., Day, M. W. & Agapie, T. A synthetic model of the Mn3Ca subsite of the oxygen-evolving complex in photosystem II. Science 333, 733–736 (2011).
Wright, R. J., Lim, C. & Tilley, T. D. Diiron proton reduction catalysts possessing electron-rich and electron-poor naphthalene-1,8-dithiolate ligands. Chem. Eur. J. 15, 8518–8525 (2009).
Lee, Y. et al. Dinitrogen activation upon reduction of a triiron(II) complex. Angew. Chem. Int. Ed. 54, 1499–1503 (2015).
Teramoto, Y., Kubo, K., Kume, S. & Mizuta, T. Formation of a hexacarbonyl diiron complex having a naphthalene-1,8-bis(phenylphosphido) bridge and the electrochemical behavior of its derivatives. Organometallics 32, 7014–7024 (2013).
Rauchfuss, T. B. Diiron azadithiolates as models for the [FeFe]-hydrogenase active site and paradigm for the role of the second coordination sphere. Acc. Chem. Res. 48, 2107–2116 (2015).
Shima, S. et al. The crystal structure of [Fe]-hydrogenase reveals the geometry of the active site. Science 321, 572–575 (2008).
Hiromoto, T. et al. The crystal structure of C176A mutated [Fe]-hydrogenase suggests an acyl-iron ligation in the active site iron complex. FEBS Lett. 583, 585–590 (2009).
Yang, X. & Hall, M. B. Trigger mechanism for the catalytic hydrogen activation by monoiron (iron−sulfur cluster-free) hydrogenase. J. Am. Chem. Soc. 130, 14036–14037 (2008).
Yang, X. & Hall, M. B. Monoiron hydrogenase catalysis: hydrogen activation with the formation of a dihydrogen, Fe−Hδ−···Hδ+−O, bond and methenyl-H4MPT+ triggered hydride transfer. J. Am. Chem. Soc. 131, 10901–10908 (2009).
Gubler, J., Finkelmann, A. R. & Reiher, M. Theoretical 57Fe Mössbauer spectroscopy for structure elucidation of [Fe] hydrogenase active site intermediates. Inorg. Chem. 52, 14205–14215 (2013).
Finkelmann, A. R., Senn, H. M. & Reiher, M. Hydrogen-activation mechanism of [Fe] hydrogenase revealed by multi-scale modeling. Chem. Sci. 5, 4474–4482 (2014).
Durgaprasad, G., Xie, Z.-L. & Rose, M. J. Iron hydride detection and intramolecular hydride transfer in a synthetic model of mono-iron hydrogenase with a CNS chelate. Inorg. Chem. 55, 386–389 (2016).
Li, B., Liu, T., Popescu, C. V., Bilko, A. & Darensbourg, M. Y. Synthesis and Mössbauer characterization of octahedral iron(II) carbonyl complexes FeI2(CO)3L and FeI2(CO)2L2: developing models of the [Fe]-H2ase active site. Inorg. Chem. 48, 11283–11289 (2009).
Royer, A. M., Salomone-Stagni, M., Rauchfuss, T. B. & Meyer-Klaucke, W. Iron acyl thiolato carbonyls: structural models for the active site of the [Fe]-hydrogenase (Hmd). J. Am. Chem. Soc. 132, 16997–17003 (2010).
Liu, T. et al. Analysis of a pentacoordinate iron dicarbonyl as synthetic analogue of the Hmd or mono-iron hydrogenase active site. Chem. Eur. J. 16, 3083–3089 (2010).
Chen, D., Scopelliti, R. & Hu, X. [Fe]-hydrogenase models featuring acylmethylpyridinyl ligands. Angew. Chem. Int. Ed. 49, 7512–7515 (2010).
Chen, D., Scopelliti, R. & Hu, X. A five-coordinate iron center in the active site of [Fe]-hydrogenase hints from a model study. Angew. Chem. Int. Ed. 50, 5671–5673 (2011).
Chen, D., Scopelliti, R. & Hu, X. Reversible protonation of a thiolate ligand in an [Fe]-hydrogenase model complex. Angew. Chem. Int. Ed. 51, 1919–1921 (2012).
Xu, T. et al. A functional model of [Fe]-hydrogenase. J. Am. Chem. Soc. 138, 3270–3273 (2016).
Song, L.-C., Xie, Z.-J., Wang, M.-M., Zhao, G.-Y. & Song, H.-B. Biomimetic models for the active site of [Fe] hydrogenase featuring an acylmethyl(hydroxymethyl)pyridine ligand. Inorg. Chem. 51, 7466–7468 (2012).
Song, L.-C., Hu, F.-Q., Zhao, G.-Y., Zhang, J.-W. & Zhang, W.-W. Several new [Fe] hydrogenase model complexes with a single Fe center ligated to an acylmethyl(hydroxymethyl)pyridine or acylmethyl(hydroxy)pyridine ligand. Organometallics 33, 6614–6622 (2014).
Turrell, P. J., Wright, J. A., Peck, J. N. T., Oganesyan, V. S. & Pickett, C. J. The third hydrogenase: a ferracyclic carbamoyl with close structural analogy to the active site of Hmd. Angew. Chem. Int. Ed. 49, 7508–7511 (2010).
Turrell, P. J., Hill, A. D., Ibrahim, S. K., Wright, J. A. & Pickett, C. J. Ferracyclic carbamoyl complexes related to the active site of [Fe]-hydrogenase. Dalton Trans. 42, 8140–8146 (2013).
Liu, T., DuBois, D. L. & Bullock, R. M. An iron complex with pendent amines as a molecular electrocatalyst for oxidation of hydrogen. Nat. Chem. 5, 228–233 (2013).
Parkin, G. Synthetic analogues relevant to the structure and function of zinc enzymes. Chem. Rev. 104, 699–768 (2004).
England, J. et al. A synthetic high-spin oxoiron(IV) complex generation, spectroscopic characterization, and reactivity. Angew. Chem. Int. Ed. 48, 3622–3626 (2009).
Dias, A. R. et al. Li, Ti(III), and Ti(IV) trisamidotriazacyclononane complexes. syntheses, reactivity, and structures. Inorg. Chem. 42, 2675–2682 (2003).
Park, Y. J., Ziller, J. W. & Borovik, A. S. The effects of redox-inactive metal ions on the activation of dioxygen: isolation and characterization of a heterobimetallic complex containing a MnIII–(μ-OH)–CaII core. J. Am. Chem. Soc. 133, 9258–9261 (2011).
Marinescu, S. C., Winkler, J. R. & Gray, H. B. Molecular mechanisms of cobalt-catalyzed hydrogen evolution. Proc. Natl Acad. Sci. USA 109, 15127–15131 (2012).
Kalz, K. F., Brinkmeier, A., Dechert, S., Mata, R. A. & Meyer, F. Functional model for the [Fe] hydrogenase inspired by the frustrated lewis pair concept. J. Am. Chem. Soc. 136, 16626–16634 (2014).
Lyon, E. J. et al. UV-A/blue-light inactivation of the ‘metal-free’ hydrogenase (Hmd) from methanogenic archaea. Eur. J. Biochem. 271, 195–204 (2004).
Acknowledgements
This research was supported by the Welch Foundation (F-1822) and the ACS Petroleum Research Fund (53542-DN13), as well as the UT College of Natural Sciences. We also thank V. Lynch for assistance with X-ray data analysis, and S. Sorey for assistance with NMR study.
Author information
Authors and Affiliations
Contributions
M.J.R. and J.S. designed the experiments. J.S. synthesized and characterized the model complexes, and J.S. performed the experiments and analysed the data. M.J.R. and J.S. carried out the DFT calculations. J.S. and T.A.M. synthesized ligands. M.J.R. and J.S. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1156 kb)
Supplementary information
Crystallographic data for compound 2. (CIF 1275 kb)
Rights and permissions
About this article
Cite this article
Seo, J., Manes, T. & Rose, M. Structural and functional synthetic model of mono-iron hydrogenase featuring an anthracene scaffold. Nature Chem 9, 552–557 (2017). https://doi.org/10.1038/nchem.2707
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2707