Abstract
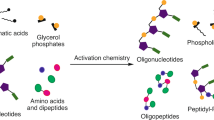
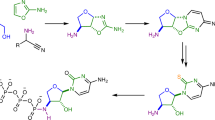
A central problem for the prebiotic synthesis of biological amino acids and nucleotides is to avoid the concomitant synthesis of undesired or irrelevant by-products. Additionally, multistep pathways require mechanisms that enable the sequential addition of reactants and purification of intermediates that are consistent with reasonable geochemical scenarios. Here, we show that 2-aminothiazole reacts selectively with two- and three-carbon sugars (glycolaldehyde and glyceraldehyde, respectively), which results in their accumulation and purification as stable crystalline aminals. This permits ribonucleotide synthesis, even from complex sugar mixtures. Remarkably, aminal formation also overcomes the thermodynamically favoured isomerization of glyceraldehyde into dihydroxyacetone because only the aminal of glyceraldehyde separates from the equilibrating mixture. Finally, we show that aminal formation provides a novel pathway to amino acids that avoids the synthesis of the non-proteinogenic α,α-disubstituted analogues. The common physicochemical mechanism that controls the proteinogenic amino acid and ribonucleotide assembly from prebiotic mixtures suggests that these essential classes of metabolite had a unified chemical origin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Eschenmoser, A. Etiology of potentially primordial biomolecular structures: from vitamin B12 to the nucleic acids and an inquiry into the chemistry of life's origin: a retrospective. Angew. Chem. Int. Ed. 50, 12412–12472 (2011).
Sutherland, J. D. The origin of life—out of the blue. Angew. Chem. Int. Ed. 55, 104–121 (2016).
Powner, M. W., Gerland, B. & Sutherland, J. D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242 (2009).
Joyce, G. F. The antiquity of RNA-based evolution. Nature 418, 214–221 (2002).
Benner, S. A., Kim, H.-Y. & Yang, Z. Setting the stage: the history, chemistry and geology behind RNA. Cold Spring Harb. Perspect. Biol. 4, a003541 (2012).
Robertson, M. P. & Joyce, G. F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 4, a003608 (2012).
Krishnamurthy, R. On the emergence of RNA. Isr. J. Chem. 55, 837–850 (2015).
Johnson, A. P. et al. The Miller volcanic spark discharge experiment. Science 322, 404 (2008).
Chyba, C. & Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature 355, 125–132 (1992).
Miller, S. L. Production of amino acids under possible primitive Earth conditions. Science 117, 528–529 (1953).
Pizzarello, S., Cooper, G. & Flynn, G. in Meteorites and the Early Solar System II (eds Lauretta, D. S. & McSween, H. Y. Jr) 625–651 (Univ. Arizona Press, 2006).
Wolman, Y., Haverland, W. J. & Miller, S. L. Nonprotein amino acids from spark discharges and their comparison with the Murchison meteorite amino acids. Proc. Natl Acad. Sci. USA 69, 809–811 (1972).
Patel, B. H., Percivalle, C., Ritson, D. J., Duffy, C. D. & Sutherland, J. D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 7, 301–307 (2015).
Powner, M. W., Zheng, S. L. & Szostak, J. W. Multicomponent assembly of proposed DNA precursors in water. J. Am. Chem. Soc. 134, 13889–13895 (2012).
Shapiro, R. Prebiotic ribose synthesis: a critical analysis. Origin Life Evol. Biosphere 18, 71–85 (1988).
Ricardo, A., Carrigan, M. A., Olcott, A. N. & Benner, S. A. Borate minerals stabilize ribose. Science 303, 196 (2004).
Breslow, R. & Cheng, Z. L. L-Amino acids catalyze the formation of an excess of D-glyceraldehyde, and thus of other D-sugars, under credible prebiotic conditions. Proc. Natl Acad. Sci. USA 107, 5723–5725 (2010).
Sagi, V. S. et al. Exploratory experiments on the chemistry of the ‘glyoxylate scenario’: formation of ketosugars from dihydroxyfumarate. J. Am. Chem. Soc. 134, 3577–3589 (2012).
Islam, S. et al. Detection of potential TNA and RNA nucleoside precursors in a prebiotic mixture by pure shift diffusion-ordered NMR spectroscopy. Chem. Eur. J. 19, 4586–4595 (2013).
Meinert, C. et al. Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science 352, 208–212 (2016).
Becker, S. et al. A high-yielding, strictly regioselective prebiotic purine nucleoside formation pathway. Science 352, 833–836 (2016).
Springsteen, G. & Joyce, G. F. Selective derivatization and sequestration of ribose from a prebiotic mix. J. Am. Chem. Soc. 126, 9578–9583 (2004).
Anastasi, C., Crowe, M. A., Powner, M. W. & Sutherland, J. D. Direct assembly of nucleoside precursors from two- and three-carbon units. Angew. Chem. Int. Ed. 45, 6176–6179 (2006).
Powner, M. W. & Sutherland, J. D. Phosphate-mediated interconversion of ribo- and arabino-configured prebiotic nucleotide intermediates. Angew. Chem. Int. Ed. 49, 4641–4643 (2010).
Powner, M. W., Sutherland, J. D. & Szostak, J. W. Chemoselective multicomponent one-pot assembly of purine precursors in water. J. Am. Chem. Soc. 132, 16677–16688 (2010).
Saul, R. et al. Reaction of 1,3-disubstituted acetone derivatives with pseudohalides: a simple approach to spiro[4.4]nonane-type bis-oxazolidines and -imidazolidines (bicyclic carbamates, thiocarbamates, ureas, and thioureas). Eur. J. Org. Chem. 1, 205–209 (2000).
Nagorski, R. W. & Richard, J. P. Mechanistic imperatives for aldose-ketose isomerization in water: specific, general base- and metal-ion-catalyzed isomerization of glyceraldehyde with proton and hydride transfer. J. Am. Chem. Soc. 123, 794–802 (2001).
Budin, I. & Szostak, J. W. Expanding roles for diverse physical phenomena during the origin of life. Annu. Rev. Biophys. 39, 245–263 (2010).
Sagan, C. & Khare, B. N. Long-wavelength ultraviolet photoproduction of amino acids on the primitive Earth. Science 173, 417–420 (1971).
Catsimpoolas, N. & Wood, J. L. Specific cleavage of cystine peptides by cyanide. J. Biol. Chem. 241, 1790–1796 (1966).
Serianni, A. S., Nunez, H. A. & Barker, R. Cyanohydrin synthesis: studies with [13C]cyanide. J. Org. Chem. 45, 3329–3341 (1980).
Schwartz, A. W. Intractable mixtures and the origin of life. Chem. Biodiv. 4, 656–664 (2007).
Schwartz, A. W. Evaluating the plausibility of prebiotic multistage syntheses. Astrobiology 13, 784–789 (2013).
Knight, R. D. & Landweber, L. F. The early evolution of the genetic code. Cell 101, 569–572 (2000).
Hein, J. E., Tse, E. & Blackmond, D. G. A route to enantiopure RNA precursors from nearly racemic starting materials. Nat. Chem. 3, 704–706 (2011).
Acknowledgements
This work was supported by the Simons Foundation (318881), the Engineering and Physical Sciences Research Council (EP/K004980/1), the Leverhulme Trust (RGP-2013-189) and through an award from the Origin of Life Challenge (M.W.P.) and a UCL Excellence Fellowship (D.-K.B.). The authors thank K. Karu for assistance with mass spectrometry and A. E. Aliev for assistance with NMR spectroscopy.
Author information
Authors and Affiliations
Contributions
M.W.P. conceived the research. M.W.P. and S.I. designed and analysed the experiments. S.I. conducted the experiments. D.-K.B. performed the crystallographic analyses. M.W.P. and S.I. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 14342 kb)
Supplementary information
Crystallographic data for compound 8a. (CIF 15 kb)
Supplementary information
Structure factors file for compound 8a. (FCF 102 kb)
Supplementary information
Crystallographic data for compound rac-8b. (CIF 15 kb)
Supplementary information
Structure factors file for compound rac-8b. (FCF 70 kb)
Supplementary information
Crystallographic data for compound D-8b. (CIF 16 kb)
Supplementary information
Structure factors file for compound D-8b. (FCF 117 kb)
Supplementary information
Crystallographic data for compound L-8b. (CIF 16 kb)
Supplementary information
Structure factors file for compound L-8b. (FCF 103 kb)
Supplementary information
Crystallographic data for compound 8c. (CIF 17 kb)
Supplementary information
Structure factors file for compound 8c. (FCF 147 kb)
Supplementary information
Crystallographic data for compound 8d. (CIF 14 kb)
Supplementary information
Structure factors file for compound 8d. (FCF 105 kb)
Supplementary information
Crystallographic data for compound 8e. (CIF 13 kb)
Supplementary information
Structure factors file for compound 8e. (FCF 51 kb)
Supplementary information
Crystallographic data for compound 8f. (CIF 15 kb)
Supplementary information
Structure factors file for compound 8f. (FCF 128 kb)
Supplementary information
Crystallographic data for compound 8g. (CIF 19 kb)
Supplementary information
Structure factors file for compound 8g. (FCF 119 kb)
Supplementary information
Crystallographic data for compound 8m. (CIF 15 kb)
Supplementary information
Structure factors file for compound 8m. (FCF 149 kb)
Supplementary information
Crystallographic data for compound D-ribo-1. (CIF 15 kb)
Supplementary information
Structure factors file for compound D-ribo-1. (FCF 71 kb)
Supplementary information
Crystallographic data for compound L-ribo-1. (CIF 15 kb)
Supplementary information
Structure factors file for compound L-ribo-1. (FCF 71 kb)
Supplementary information
Crystallographic data for compound rac-ribo-1. (CIF 14 kb)
Supplementary information
Structure factors file for compound rac-ribo-1. (FCF 71 kb)
Supplementary information
Crystallographic data for compound rac-threo-5. (CIF 26 kb)
Supplementary information
Structure factors file for compound rac-threo-5. (FCF 299 kb)
Rights and permissions
About this article
Cite this article
Islam, S., Bučar, DK. & Powner, M. Prebiotic selection and assembly of proteinogenic amino acids and natural nucleotides from complex mixtures. Nature Chem 9, 584–589 (2017). https://doi.org/10.1038/nchem.2703
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2703
This article is cited by
-
Heat flows enrich prebiotic building blocks and enhance their reactivity
Nature (2024)
-
Thiophosphate photochemistry enables prebiotic access to sugars and terpenoid precursors
Nature Chemistry (2023)
-
Chirality-induced avalanche magnetization of magnetite by an RNA precursor
Nature Communications (2023)
-
Prebiotic synthesis and triphosphorylation of 3′-amino-TNA nucleosides
Nature Chemistry (2022)
-
Charge-density reduction promotes ribozyme activity in RNA–peptide coacervates via RNA fluidization and magnesium partitioning
Nature Chemistry (2022)