Abstract
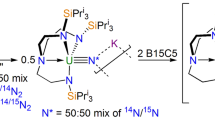
Our knowledge of actinide chemical bonds lags far behind our understanding of the bonding regimes of any other series of elements. This is a major issue given the technological as well as fundamental importance of f-block elements. Some key chemical differences between actinides and lanthanides—and between different actinides—can be ascribed to minor differences in covalency, that is, the degree to which electrons are shared between the f-block element and coordinated ligands. Yet there are almost no direct measures of such covalency for actinides. Here we report the first pulsed electron paramagnetic resonance spectra of actinide compounds. We apply the hyperfine sublevel correlation technique to quantify the electron-spin density at ligand nuclei (via the weak hyperfine interactions) in molecular thorium(III) and uranium(III) species and therefore the extent of covalency. Such information will be important in developing our understanding of the chemical bonding, and therefore the reactivity, of actinides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Morss, L. R., Edelstein, N., Fuger, J. & Katz, J. J. (eds) The Chemistry of the Actinide and Transactinide Elements 4th edn (Springer, 2010).
Natrajan, L. S. & Langford-Paden, M. H. in Element Recovery and Sustainability (ed. Hunt, A.) Ch. 6 (Royal Society of Chemistry, 2013).
Kozimor, S. A. et al. Trends in covalency for d- and f-element metallocene dichlorides identified using chlorine K-edge X-ray absorption spectroscopy and time-dependent density functional theory. J. Am. Chem. Soc. 131, 12125–12136 (2009).
Minasian, S. G. et al. Determining relative f and d orbital contributions to M–Cl covalency in (M = Ti, Zr, Hf, U) and using Cl K-edge X-ray absorption spectroscopy and time-dependent density functional theory. J. Am. Chem. Soc. 134, 5586–5597 (2012).
Polinski, M. J. et al. Differentiating between trivalent lanthanides and actinides. J. Am. Chem. Soc. 134, 10682–10692 (2012).
Jones, M. B. et al. Uncovering f-element bonding differences and electronic structure in a series of 1:3 and 1:4 complexes with a diselenophosphate ligand. Chem. Sci. 4, 1189–1203 (2013).
Minasian, S. G. et al. New evidence for 5f covalency in actinocenes determined from carbon K-edge XAS and electronic structure theory. Chem. Sci. 5, 351–359 (2014).
Cary, S. K. et al. Emergence of californium as the second transitional element in the actinide series. Nat. Commun. 6, 6827 (2015).
Liddle, S. T. The renaissance of non-aqueous uranium chemistry. Angew. Chem. Int. Ed. 54, 8604–8641 (2015).
Dutkiewicz, M. S. et al. Organometallic neptunium(III) complexes. Nat. Chem. 8, 797–802 (2016).
Silver, M. A. et al. Characterization of berkelium(III) dipicolinate and borate compounds in solution and the solid state. Science 353, 3762–3763 (2016).
Neidig, M. L., Clark, D. L. & Martin, L. R. Covalency in f-element complexes. Coord. Chem. Rev. 257, 394–406 (2013).
Kaltsoyannis, N. Does covalency increase or decrease across the actinide series? Implications for minor actinide partitioning. Inorg. Chem. 52, 3407–3413 (2013).
Gagliardi, L. & Roos, B. O. Multiconfigurational quantum chemical methods for molecular systems containing actinides. Chem. Soc. Rev. 36, 893–903 (2007).
Gagliardi, L. The study of actinide chemistry with multiconfigurational quantum chemical methods. Int. J. Quant. Chem. 111, 3302–3306 (2011).
Wang, D., van Gunsteren, W. F. & Chai, Z. Recent advances in computational actinoid chemistry. Chem. Soc. Rev. 41, 5836–5865 (2012).
Averkiev, B. B. et al. How accurate are electronic structure methods for actinoid chemistry? Theor. Chem. Acc. 129, 657–666 (2011).
Choppin, G. R. Covalency in f-element bonds. J. Alloys Compd 344, 55–59 (2002).
Solomon, E. I. & Lever, A. B. P. (eds) Inorganic Structure and Spectroscopy (Wiley, 1999).
Denecke, M. A. Synchrotron applications to f-element research in the nuclear fuel cycle. Dalton Trans. 44, 2606–2612 (2015).
Solomon, E. I., Hedman, B., Hodgson, K. O., Dey, A. & Szilagyi, R. K. Ligand K-edge X-ray absorption spectroscopy: covalency of ligand–metal bonds. Coord. Chem. Rev. 249, 97–129 (2005).
Smiles, D. E., Wu, G., Hrobárik, P. & Hayton, T. W. Use of 77Se and 125Te NMR spectroscopy to probe covalency of the actinide–chalcogen bonding in [Th(En){N(SiMe3)2}3]– (E = Se, Te; n = 1, 2) and their oxo-uranium(VI) congeners. J. Am. Chem. Soc. 138, 814–825 (2016).
Boatner, L. A. & Abraham, M. M. Electron paramagnetic resonance from actinide elements. Rep. Prog. Phys. 41, 87–155 (1978).
Aminov, L. K., Kurkin, I. N. & Malkin, B. Z. Superhyperfine structure in the EPR spectra and optical spectra of impurity f ions in dielectric crystals: a review. Phys. Sol. State 55, 1343–1363 (2013).
Bleaney, B., Llewellyn, P. M. & Jones, D. A. Paramagnetic resonance of uranium ions. Proc. Phys. Soc. B 69, 858–860 (1956).
Kolbe, W. & Edelstein, N. Electron-nuclear double resonance of Pu3+ in CaF2 . Phys. Rev. B 4, 2869–2875 (1971).
Gourier, D., Caurant, D., Arliguie, T. & Ephritikhine, M. EPR and angle-selected ENDOR study of 5f-ligand interactions in the [U(η7-C7H7)2]– anion, an f1 analogue of uranocene. J. Am. Chem. Soc. 120, 6084–6092 (1998).
Kiel, A. & Mims, W. B. Linear electric field effect in paramagnetic resonance for Nd3+ and U3+ tetragonal sites in fluorite lattices. Phys. Rev. B 7, 2917–2919 (1973).
Nugent, L. J. et al. Noncovalent character in the chemical bonds of the lanthanide(III) and the actinide(III) tricyclopentadienides. J. Organomet. Chem. 27, 365–372 (1971).
Strittmatter, R. J. & Bursten, B. E. Bonding in tris(η5-cyclopentadienyl) actinide complexes. 5. A comparison of the bonding in Np, Pu, and transplutonium compounds with that in lanthanide compounds and a transition metal analogue. J. Am. Chem. Soc. 113, 552–559 (1991).
Bursten, B. E., Rhodes, L. F. & Strittmatter, R. J. Bonding in tris(η5-cyclopentadienyl) actinide complexes. 2. On the ground electronic configurations of ‘base-free’ Cp3An complexes (An = Th, Pa, U, Np, Pu). J. Am. Chem. Soc. 111, 2756–2758 (1989).
Kaltsoyannis, N. & Bursten, B. E. Electronic structure of f1 lanthanide and actinide complexes. Part 2. Non-relativistic and relativistic calculations of the ground state electronic structures and optical transition energies of [Ce(η-C5H5)3], [Th(η-C5H5)3] and [Pa(η-C5H5)3]. J. Organomet. Chem. 528, 19–33 (1997).
Kirker, I. & Kaltsoyannis, N. Does covalency really increase across the 5f series? A comparison of molecular orbital, natural population, spin and electron density analyses of AnCp3 (An = Tm–Cm; Cp = η5-C5H5). Dalton Trans. 40, 124–131 (2011).
Denning, R. G., Harmer, J., Green, J. C. & Irwin, M. Covalency in the 4f shell of tris-cyclopentadienyl ytterbium (YbCp3)—a spectroscopic investigation. J. Am. Chem. Soc. 133, 20644–20660 (2011).
Langeslay, R. R., Fieser, M. E., Ziller, J. W., Furche, F. & Evans, W. J. Synthesis, structure, and reactivity of crystalline molecular complexes of the {[C5H3(SiMe3)2]3Th}1– anion containing thorium in the formal +2 oxidation state. Chem. Sci. 6, 517–521 (2015).
Blake, P. C. et al. Synthesis, properties and structures of the tris(cyclopentadienyl)thorium(III) complexes [Th{η5-C5H3(SiMe2R)2-1,3}3] (R = Me or tBu). J. Organomet. Chem. 636, 124–129 (2001).
Siladke, N. A. et al. Actinide metallocene hydride chemistry: C–H activation in tetramethylcyclopentadienyl ligands to form [μ-η5-C5Me3H(CH2)-κC]2– tuck-over ligands in a tetrathorium octahydride complex. Organometallics 32, 6522–6531 (2013).
Blake, P. C., Lappert, M. F., Atwood, J. L. & Zhang, H. The synthesis and characterisation, including X-ray diffraction study, of [Th{η-C5H3(SiMe3)2}3]; the first thorium(III) crystal structure. J. Chem. Soc. Chem. Commun. 1148–1149 (1986).
Kot, W. K., Shalimoff, G. V., Edelstein, N. M., Edelman, M. A. & Lappert, M. F. [ThIII{η5-C5H3(SiMe3)2}3], an actinide compound with a 6d1 ground state. J. Am. Chem. Soc. 110, 986–987 (1988).
Lukens, W. W. et al. The roles of 4f- and 5f-orbitals in bonding: a magnetochemical, crystal field, density functional theory, and multi-reference wavefunction study. Dalton Trans. 45, 11508–11521 (2016).
Tassell, M. J. & Kaltsoyannis, N. Covalency in AnCp4 (An = Th–Cm): a comparison of molecular orbital, natural population and atoms-in-molecules analyses. Dalton Trans. 39, 6719–6725 (2010).
Schweiger, A. & Jeschke, J. Principles of Pulsed Electron Paramagnetic Resonance (Oxford Univ. Press, 2001).
Stoll, S. & Britt, R. D. General and efficient simulation of pulse EPR spectra. Phys. Chem. Chem. Phys. 11, 6614–6625 (2009).
Atherton, N. M. Principles of Electron Spin Resonance (Ellis Horwood, 1993).
Ren, W., Zhao, N., Chen, L., Song, H. & Zi, G. Synthesis, structure and catalytic activity of an organothorium hydride complex. Inorg. Chem. Commun. 14, 1838–1841 (2011).
Acknowledgements
This paper is dedicated to D. Gatteschi (University of Florence) on the occasion of his retirement. We acknowledge the Engineering and Physical Sciences Research Council (grant no. EP/K039547/1, EP/L014416/1 and EP/J002208/1), the Nuclear FiRST DTC (doctoral scholarship to A.F.), Marie Curie Actions, The European Union (FP7-PEOPLE-2013-ITN ‘MAGIC’ Initial Training Network for a doctoral scholarship to A.-M.A.) and The University of Manchester, University College London, Lancaster University and the UK National EPR Facility and Service for supporting this work.
Author information
Authors and Affiliations
Contributions
A.F. synthesized and characterized the compounds. F.O. carried out the single-crystal X-ray diffraction analysis. A.-M.A., F.T. and E.J.L.M. collected and interpreted the EPR spectroscopy and magnetic data. R.B. and A.K. performed and interpreted the calculations. D.P.M. provided the initial concept and supervised A.F., D.P.M. and E.J.L.M. wrote the manuscript, with contributions from all the co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 6336 kb)
Supplementary information
CrystalData.cif Crystallographic data for compound 1, 2 and ([U(Cptt)3(Cl)]) (CIF 2736 kb)
Rights and permissions
About this article
Cite this article
Formanuik, A., Ariciu, AM., Ortu, F. et al. Actinide covalency measured by pulsed electron paramagnetic resonance spectroscopy. Nature Chem 9, 578–583 (2017). https://doi.org/10.1038/nchem.2692
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2692
This article is cited by
-
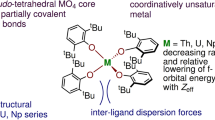
Bonding of isovalent homologous actinide and lanthanide pairs with chalcogenide donors: effect of metal f-orbital participation and donor softness
Structural Chemistry (2023)
-
Covalent bond shortening and distortion induced by pressurization of thorium, uranium, and neptunium tetrakis aryloxides
Nature Communications (2022)
-
Measuring molecular magnets for quantum technologies
Nature Reviews Physics (2021)
-
Exceptional uranium(VI)-nitride triple bond covalency from 15N nuclear magnetic resonance spectroscopy and quantum chemical analysis
Nature Communications (2021)
-
Isolation and characterization of a californium metallocene
Nature (2021)