Abstract
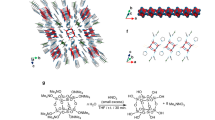
Two-dimensional covalent organic frameworks often π stack into crystalline solids that allow precise spatial positioning of molecular building blocks. Inspired by the hydrogen-bonded G-quadruplexes found frequently in guanine-rich DNA, here we show that this structural motif can be exploited to guide the self-assembly of naphthalene diimide and perylene diimide electron acceptors end-capped with two guanine electron donors into crystalline G-quadruplex-based organic frameworks, wherein the electron donors and acceptors form ordered, segregated π-stacked arrays. Time-resolved optical and electron paramagnetic resonance spectroscopies show that photogenerated holes and electrons in the frameworks have long lifetimes and display recombination kinetics typical of dissociated charge carriers. Moreover, the reduced acceptors form polarons in which the electron is shared over several molecules. The G-quadruplex frameworks also demonstrate potential as cathode materials in Li-ion batteries because of the favourable electron- and Li-ion-transporting capacity provided by the ordered rylene diimide arrays and G-quadruplex structures, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Coropceanu, V. et al. Charge transport in organic semiconductors. Chem. Rev. 107, 926–952 (2007).
Grozema, F. C. & Siebbeles, L. D. A. Mechanism of charge transport in self-organizing organic materials. Int. Rev. Phys. Chem. 27, 87–138 (2008).
Roznyatovskiy, V. V., Carmieli, R., Dyar, S. M., Brown, K. E. & Wasielewski, M. R. Photodriven charge separation and transport in self-assembled zinc tetrabenzotetraphenylporphyrin and perylenediimide charge conduits. Angew. Chem. Int. Ed. 53, 3457–3461 (2014).
Hartnett, P. E. et al. Long-lived charge carrier generation in ordered films of a covalent perylenediimide-diketopyrrolopyrrole-perylenediimide molecule. Chem. Sci. 6, 402–411 (2015).
Mallia, A. R., Salini, P. S. & Hariharan, M. Nonparallel stacks of donor and acceptor chromophores evade geminate charge recombination. J. Am. Chem. Soc. 137, 15604–15607 (2015).
Dogru, M. & Bein, T. On the road towards electroactive covalent organic frameworks. Chem. Commun. 50, 5531–5546 (2014).
Colson, J. W. & Dichtel, W. R. Rationally synthesized two-dimensional polymers. Nat. Chem. 5, 453–465 (2013).
Ding, S.-Y. & Wang, W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013).
Waller, P. J., Gandara, F. & Yaghi, O. M. Chemistry of covalent organic frameworks. Acc. Chem. Res. 48, 3053–3063 (2015).
Wan, S., Guo, J., Kim, J., Ihee, H. & Jiang, D. A photoconductive covalent organic framework: self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chem. Int. Ed. 48, 5439–5442 (2009).
Jin, S. B. et al. Charge dynamics in a donor–acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions. Angew. Chem. Int. Ed. 52, 2017–2021 (2013).
Wan, S. et al. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 23, 4094–4097 (2011).
Ding, X. S. et al. Synthesis of metallophthalocyanine covalent organic frameworks that exhibit high carrier mobility and photoconductivity. Angew. Chem. Int. Ed. 50, 1289–1293 (2011).
Cai, S.-L. et al. Tunable electrical conductivity in oriented thin films of tetrathiafulvalene-based covalent organic framework. Chem. Sci. 5, 4693–4700 (2014).
Wilson, A. J. Non-covalent polymer assembly using arrays of hydrogen-bonds. Soft Matter 3, 409–425 (2007).
Chen, T.-H. et al. Thermally robust and porous noncovalent organic framework with high affinity for fluorocarbons and CFCs. Nat. Commun. 5, 5131 (2014).
Li, P. et al. A rod-packing microporous hydrogen-bonded organic framework for highly selective separation of C2H2/CO2 at room temperature. Angew. Chem. Int. Ed. 54, 574–577 (2015).
He, Y. B., Xiang, S. C. & Chen, B. L. A microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature. J. Am. Chem. Soc. 133, 14570–14573 (2011).
Wu, Y.-L., Brown, K. E. & Wasielewski, M. R. Extending photoinduced charge separation lifetimes by using supramolecular design: guanine–perylenediimide G-quadruplex. J. Am. Chem. Soc. 135, 13322–13325 (2013).
Wu, Y.-L., Brown, K. E., Gardner, D. M., Dyar, S. M. & Wasielewski, M. R. Photoinduced hole injection into a self-assembled π-extended G-quadruplex. J. Am. Chem. Soc. 137, 3981–3990 (2015).
Sessler, J. L., Sathiosatham, M., Doerr, K., Lynch, V. & Abboud, K. A. A G-quartet formed in the absence of a templating metal cation: a new 8-(N,N-dimethylaniline)guanosine derivative. Angew. Chem. Int. Ed. 39, 1300–1303 (2000).
Otero, R. et al. Guanine quartet networks stabilized by cooperative hydrogen bonds. Angew. Chem. Int. Ed. 44, 2270–2275 (2005).
Zambounis, J. S., Hao, Z. & Iqbal, A. Latent pigments activated by heat. Nature 388, 131–132 (1997).
Hartnett, P. E. et al. Slip-stacked perylenediimides as an alternative strategy for high efficiency nonfullerene acceptors in organic photovoltaics. J. Am. Chem. Soc. 136, 16345–16356 (2014).
Huang, N., Chen, X., Krishna, R. & Jiang, D. L. Two-dimensional covalent organic frameworks for carbon dioxide capture through channel-wall functionalization. Angew. Chem. Int. Ed. 54, 2986–2990 (2015).
Giorgi, T. et al. Gel-like lyomesophases formed in organic solvents by self-assembled guanine ribbons. Chem. Eur. J. 8, 2143–2152 (2002).
El Garah, M. et al. Guanosine-based hydrogen-bonded 2D scaffolds: metal-free formation of G-quartet and G-ribbon architectures at the solid/liquid interface. Chem. Commun. 51, 11677–11680 (2015).
Wu, Y. et al. Electron delocalization in a rigid cofacial naphthalene-1,8:4,5-bis(dicarboximide) dimer. Angew. Chem. Int. Ed. 53, 9476–9481 (2014).
McCarthy, B. D., Hontz, E. R., Yost, S. R., Van Voorhis, T. & Dinca, M. Charge transfer or J-coupling? Assignment of an unexpected red-shifted absorption band in a naphthalenediimide-based metal–organic framework. J. Phys. Chem. Lett. 4, 453–458 (2013).
Yushchenko, O. et al. Ultrafast intersystem-crossing dynamics and breakdown of the Kasha-Vavilov's rule of naphthalenediimides. J. Phys. Chem. Lett. 6, 2096–2100 (2015).
Bullock, J. E. et al. Photophysics and redox properties of rylene imide and diimide dyes alkylated ortho to the imide groups. J. Phys. Chem. B 114, 1794–1802 (2010).
Gosztola, D., Niemczyk, M. P., Svec, W., Lukas, A. S. & Wasielewski, M. R. Excited doublet states of electrochemically generated aromatic imide and diimide radical anions. J. Phys. Chem. A 104, 6545–6551 (2000).
Weller, A. Photoinduced electron-transfer in solution—exciplex and radical ion-pair formation free enthalpies and their solvent dependence. Z. Phys. Chem. 133, 93–98 (1982).
Credgington, D., Jamieson, F. C., Walker, B., Nguyen, T. Q. & Durrant, J. R. Quantification of geminate and non-geminate recombination losses within a solution-processed small-molecule bulk heterojunction solar cell. Adv. Mater. 24, 2135–2141 (2012).
Hodgkiss, J. M. et al. Subnanosecond geminate charge recombination in polymer–polymer photovoltaic devices. Phys. Rev. Lett. 104, 177701 (2010).
Wiederrecht, G. P., Svec, W. A. & Wasielewski, M. R. Triplet states with unusual spin polarization resulting from radical ion pair recombination at short distances. J. Am. Chem. Soc. 121, 7726–7727 (1999).
Bullock, J. E., Carmieli, R., Mickley, S. M., Vura-Weis, J. & Wasielewski, M. R. Photoinitiated charge transport through π-stacked electron conduits in supramolecular ordered assemblies of donor−acceptor triads. J. Am. Chem. Soc. 131, 11919–11929 (2009).
Closs, G. L., Forbes, M. D. E. & Norris, J. R. Spin-polarized electron-paramagnetic resonance-spectra of radical pairs in micelles—observation of electron spin–spin interactions. J. Phys. Chem. 91, 3592–3599 (1987).
Kobori, Y., Sekiguchi, S., Akiyama, K. & Tero-Kubota, S. Chemically induced dynamic electron polarization study on the mechanism of exchange interaction in radical ion pairs generated by photoinduced electron transfer reactions. J. Phys. Chem. A 103, 5416–5424 (1999).
Trifunac, A. D., Nelson, D. J. & Mottley, C. Chemically induced dynamic electron polarization. Examples of S–T±1 polarization. J. Magn. Reson. 30, 263–272 (1978).
Nardes, A. M. et al. Photoinduced charge carrier generation and decay in sequentially deposited polymer/fullerene layers: bulk heterojunction vs planar interface. J. Phys. Chem. C 116, 7293–7305 (2012).
Ferguson, A. J., Kopidakis, N., Shaheen, S. E. & Rumbles, G. Dark carriers, trapping, and activation control of carrier recombination in neat P3HT and P3HT:PCBM blends. J. Phys. Chem. C 115, 23134–23148 (2011).
Mondal, T., Sakurai, T., Yoneda, S., Seki, S. & Ghosh, S. Semiconducting nanotubes by intrachain folding following macroscopic assembly of a naphthalene–diimide (NDI) appended polyurethane. Macromolecules 48, 879–888 (2015).
Norris, J. R., Uphaus, R. A., Crespi, H. L. & Katz, J. Electron spin resonance of chlorophyll and origin of signal-I in photosynthesis. Proc. Natl Acad. Sci. USA 68, 625–628 (1971).
Song, Z. & Zhou, H. Towards sustainable and versatile energy storage devices: an overview of organic electrode materials. Energy Environ. Sci. 6, 2280–2301 (2013).
Liang, Y., Tao, Z. & Chen, J. Organic electrode materials for rechargeable lithium batteries. Adv. Ener. Mater. 2, 742–769 (2012).
Song, Z., Zhan, H. & Zhou, Y. Polyimides: promising energy-storage materials. Angew. Chem. Int. Ed. 49, 8444–8448 (2010).
Xu, F. et al. Electrochemically active, crystalline, mesoporous covalent organic frameworks on carbon nanotubes for synergistic lithium-ion battery energy storage. Sci. Rep. 5, 8225 (2015).
Forman, S. L., Fettinger, J. C., Pieraccini, S., Gottareli, G. & Davis, J. T. Toward artificial ion channels: a lipophilic G-quadruplex. J. Am. Chem. Soc. 122, 4060–4067 (2000).
Arnal-Herault, C. et al. Functional G-quartet macroscopic membrane films. Angew. Chem. Int. Ed. 46, 8409–8413 (2007).
Acknowledgements
This work was supported by the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Department of Energy (DOE) under grant no. DE-FG02-99ER14999 (M.R.W.). N.E.H. was supported in part by the Department of Energy Office of Science Graduate Fellowship Program (DOE SCGF), made possible in part by the American Recovery and Reinvestment Act of 2009, administered by ORISE-ORAU under contract no. DE-AC05-06OR23100. K.-S.C. and M.C.H. acknowledge the Center for Electrochemical Energy Science, an Energy Frontier Research Center funded by the US DOE Office of Basic Energy Sciences (Award No. DE-AC02-06CH11357), for Li-ion battery fabrication and testing. The computational work was supported by the National Science Foundation (NSF) under grant no. DMR-1334928 (R.Q.S.). J.T.H. and O.K.F. acknowledge support from the US DOE Office of Science, Office of Basic Energy Sciences (grant no. DE-FG02-87ER13808) and Northwestern University. This work made use of the J.B. Cohen X-Ray Diffraction Facility supported by the MRSEC program of the National Science Foundation (DMR-1121262) at the Materials Research Center of Northwestern University and the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF NNCI-1542205). This work made use of the EPIC facility of the NUANCE Center at Northwestern University, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF NNCI-1542205); the MRSEC program (NSF DMR-1121262) at the Materials Research Center; the International Institute for Nanotechnology (IIN); the Keck Foundation; and the State of Illinois, through the IIN.
Author information
Authors and Affiliations
Contributions
Y.-L.W. and M.R.W. planned and directed the project. Y.-L.W., N.E.H., N.S.L., K.-S.C., L.M, and T.C.W. prepared the materials, performed the structural characterization and carried out measurements and data analysis. D.A.G.-G. developed the structural models and carried out the simulations. All the authors contributed to the analysis of the results and the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5547 kb)
Rights and permissions
About this article
Cite this article
Wu, YL., Horwitz, N., Chen, KS. et al. G-quadruplex organic frameworks. Nature Chem 9, 466–472 (2017). https://doi.org/10.1038/nchem.2689
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2689
This article is cited by
-
Chalcogen-bridged coordination polymer for the photocatalytic activation of aryl halides
Nature Communications (2023)
-
Hydrogen-bonded organic framework for red light-mediated photocatalysis
Nano Research (2023)
-
An aqueous rechargeable zinc-ion battery on basis of an organic pigment
Rare Metals (2022)
-
Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion
Nature Communications (2019)
-
The art of two-dimensional soft nanomaterials
Science China Chemistry (2019)