Abstract
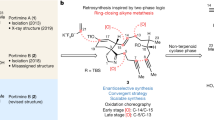
Tumour hypoxia is speculated to be a key driver of therapeutic resistance and metastatic dissemination. Consequently, the discovery of new potent agents that selectively target the hypoxic cell population may reveal new and untapped antitumour mechanisms. Here we demonstrate that the BE-43547 subclass of the APD-CLD (amidopentadienoate-containing cyclolipodepsipeptides) natural products possesses highly hypoxia-selective growth-inhibitory activity against pancreatic cancer cells. To enable this discovery, we have developed the first synthesis of the BE-43547-macrocyclic scaffold in 16 steps (longest linear sequence), which also allowed access to the full panel of relative stereoisomers and ultimately to the assignment of stereochemical configuration. Discrepancies between the spectroscopic signatures of the synthetic compounds with that originally reported for the BE-43547 members stimulated us to re-isolate the natural product from a BE-43547-producing microorganism during which we elucidated the biosynthetic gene clusters for the BE-43547 family as well as for all other known APD-CLDs. Our studies underline the exciting possibilities for the further development of the anticancer activities of these natural products.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Walsh, C. T. & Fischbach, M. A. Natural products version 2.0: connecting genes to molecules. J. Am. Chem. Soc. 132, 2469–2493 (2010).
Winter, J. M., Behnken, S. & Hertweck, C. Genomics-inspired discovery of natural products. Curr. Opin. Chem. Biol. 15, 22–31 (2011).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661 (2016).
Harvey, A. L., Edrada-Ebel, R. & Quinn, R. J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 14, 111–129 (2015).
Nishioka, H., Nakajima, S., Nagashima, M., Kojiri, K. & Suda, H. BE-43547 series substances, their manufacture with Streptomyces species, and their use as antitumor agent. Japanese patent 10147594 A 19980602 (1998).
McBrien, K. D. et al. Rakicidins, new cytotoxic lipopeptides from Micromonospora sp. fermentation, isolation and characterization. J. Antibiot. 48, 1446–1452 (1995).
Yamazaki, Y., Kunimoto, S. & Ikeda, D. Rakicidin A: a hypoxia-selective cytotoxin. Biol. Pharm. Bull. 30, 261–265 (2007).
Takeuchi, M. et al. Rakicidin A effectively induces apoptosis in hypoxia adapted Bcr-Abl positive leukemic cells. Cancer Sci. 102, 591–596 (2011).
Poulsen, T. B. A concise route to the macrocyclic core of the rakicidins. Chem. Commun. 47, 12837–12839 (2011).
Sang, F. et al. Total synthesis and determination of the absolute configuration of rakicidin A. J. Am. Chem. Soc. 136, 15787–15791 (2014).
Oku, N. et al. Complete stereochemistry and preliminary structure–activity relationship of rakicidin A, a hypoxia-selective cytotoxin from Micromonospora sp. J. Nat. Prod. 77, 2561–2565 (2014).
Clement, L. L. et al. The amido-pentadienoate-functionality of the rakicidins is a thiol reactive electrophile—development of a general synthetic strategy. Chem. Commun. 51, 12427–12430 (2015).
Tsakos, M. et al. Total synthesis and biological evaluation of rakicidin A and discovery of a simplified bioactive analogue. Angew. Chem. Int. Ed. 55, 1030–1035 (2016).
Hu, J.-F. et al. Rakicidin C, a new cyclic depsipeptide from Streptomyces sp. Eur. J. Org. Chem. 3353–3356 (2000).
Igarashi, Y. et al. Rakicidin D, an inhibitor of tumor cell invasion from marine-derived Streptomyces sp. J. Antibiot. 63, 563–565 (2010).
Igarashi, M. et al. Vinylamycin, a new depsipeptide antibiotic, from Streptomyces sp. J. Antibiot. 52, 873–879 (1999).
Yang, Z. et al. Total synthesis and determination of the absolute configuration of vinylamycin. Org. Lett. 17, 5725–5727 (2015).
Carr, G. et al. Microtermolides A and B from termite-associated Streptomyces sp. and structural revision of vinylamycin. Org. Lett. 14, 2822–2825 (2012).
Yang, Z. et al. Determination of the absolute configurations of microtermolides A and B. J. Nat. Prod. 79, 2408–2412 (2016).
Tsakos, M., Jacobsen, K. M., Yu, W., Villadsen, N. L. & Poulsen, T. B. The rakicidin family of anticancer natural products—synthetic strategies towards a new class of hypoxia-selective cytotoxins. Synlett. 27, 1898–1906 (2016).
Shirokawa, S.-I. et al. Remote asymmetric induction with vinylketene silyl N,O-acetal. J. Am. Chem. Soc. 126, 13604–13605 (2004).
Mukaeda, Y., Kato, T. & Hosokawa, S. Syn-selective Kobayashi aldol reaction using the E,E-vinylketene silyl N,O-acetal. Org. Lett. 14, 5298–5301 (2012).
Pittelkow, M., Kamounah, F. S., Boas, U., Pedersen, B. & Christensen, J. B. TFFH as an excellent reagent for acylation of alcohols, thiols and dithiocarbamates. Synthesis 2482–2492 (2004).
Tsakos, M., Schaffert, E. S., Clement, L. L., Villadsen, N. L. & Poulsen, T. B. Ester coupling reactions—an enduring challenge in the chemical synthesis of bioactive natural products. Nat. Prod. Rep. 32, 605–632 (2015).
Bennani, Y. L. & Sharpless, K. B. Asymmetric dihydroxylation (AD) of N,N-dialkyl and N-methoxy-N-methyl α,β- and β,γ-unsaturated amides. Tetrahedron Lett. 34, 2079–2082 (1993).
Dupau, P., Epple, R., Thomas, A. A., Fokin, V. V. & Sharpless, K. B. Osmium-catalyzed dihydroxylation of olefins in acidic media: old process, new tricks. Adv. Synth. Catal. 344, 421–433 (2002).
Plietker, B. RuO4-catalyzed ketohydroxylation of olefins. J. Org. Chem. 68, 7123–7125 (2003).
Ozanne, A., Pouységu, L., Depernet, D., François, B. & Quideau, S. A stabilized formulation of IBX (sIBX) for safe oxidation reactions including a new oxidative demethylation of phenolic methyl aryl ethers. Org. Lett. 5, 2903–2906 (2003).
Schauer, D. & Helquist, P. Mild zinc-promoted Horner–Wadsworth–Emmons reactions of diprotic phosphonate reagents. Synthesis 3654–3660 (2006).
Albright, J. C., Goering, A. W., Doroghazi, J. R., Metcalf, W. W. & Kelleher, N. L. Strain-specific proteogenomics accelerates the discovery of natural products via their biosynthetic pathways. J. Ind. Microbiol. Biotechnol. 41, 451–459 (2014).
Waters, A. L. et al. An analysis of the sponge Acanthostrongylophora igens’ microbiome yields an actinomycete that produces the natural product manzamine A. Front. Mar. Sci. 1, 6919 (2014).
Abdelmohsen, U. R. et al. Actinomycetes from Red Sea sponges: sources for chemical and phylogenetic diversity. Mar. Drugs 12, 2771–2789 (2014).
Jensen, P. R. & Mafnas, C. Biogeography of the marine actinomycete Salinispora. Environ. Microbiol. 8, 1881–1888 (2006).
Weissman, K. J. et al. The molecular basis of Celmer's rules: the stereochemistry of the condensation step in chain extension on the erythromycin polyketide synthase. Biochemistry 36, 13849–13855 (1997).
Valenzano, C. R., Lawson, R. J., Chen, A. Y., Khosla, C. & Cane, D. E. The biochemical basis for stereochemical control in polyketide biosynthesis. J. Am. Chem. Soc. 131, 18501–18511 (2009).
Zheng, J. & Keatinge-Clay, A. T. Structural and functional analysis of C2-type ketoreductases from modular polyketide synthases. J. Mol. Biol. 410, 105–117 (2011).
Jensen, F., Unifying general and segmented contracted basis sets. Segmented polarization consistent basis sets. J. Chem. Theory Comput. 10, 1074–1085 (2014).
Chai, J.-D. & Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008).
Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 4, 437–447 (2004).
Rankin, E. B. & Giaccia, A. J. Hypoxic control of metastasis. Science 352, 175–180 (2016).
Demain, A. L. & Fang, A. The natural functions of secondary metabolites. Adv. Biochem. Eng. Biotechnol. 69, 1–39 (2000).
Acknowledgements
We thank the laboratories of M. T. Hamann and R. T. Hill for providing Micromonospora sp. Strain M42, U. R. Abdelmohsen and U. Hentschel Humeida for providing Micromonospora sp. Strain RV43 and P. Jensen for providing Salinospora arenicola CNR107. C. Bak Nielsen is acknowledged for technical assistance. T.B.P. gratefully acknowledges support from the Lundbeck Foundation (fellow grant R105-A9308) and the Carlsberg Foundation (grant CF15-0431). T.T. gratefully acknowledges support from the Danish Council for Independent Research (DFF – 4181-00315).
Author information
Authors and Affiliations
Contributions
T.B.P. conceived and supervised the study, T.B.P., T.T. and N.L.V. designed the experiments. N.L.V., E.T.W., B.C. and U.B.K. performed the organic synthesis. K.M.J. conducted cytotoxicity experiments. N.L.V. and T.T. performed the bacterial fermentations and isolations, T.T. conducted the bioinformatic studies, U.B.K. and F.J. performed the computational studies. M.J., M.B. and T.V. helped with MS and NMR analyses. T.B.P., T.T. and N.L.V. wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 15068 kb)
Rights and permissions
About this article
Cite this article
Villadsen, N., Jacobsen, K., Keiding, U. et al. Synthesis of ent-BE-43547A1 reveals a potent hypoxia-selective anticancer agent and uncovers the biosynthetic origin of the APD-CLD natural products. Nature Chem 9, 264–272 (2017). https://doi.org/10.1038/nchem.2657
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2657
This article is cited by
-
Discovery of a nitroaromatic nannocystin with potent in vivo anticancer activity against colorectal cancer by targeting AKT1
Acta Pharmacologica Sinica (2024)
-
Development of a drug discovery approach from microbes with a special focus on isolation sources and taxonomy
The Journal of Antibiotics (2023)
-
Dual hypoxia-responsive supramolecular complex for cancer target therapy
Nature Communications (2023)
-
Comparative genome features and secondary metabolite biosynthetic potential of Kutzneria chonburiensis and other species of the genus Kutzneria
Scientific Reports (2023)
-
Bioactive natural products from the genus Salinospora: a review
Archives of Pharmacal Research (2020)