Abstract
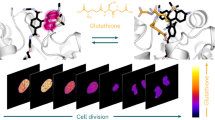
Alterations in glutathione (GSH) homeostasis are associated with a variety of diseases and cellular functions, and therefore, real-time live-cell imaging and quantification of GSH dynamics are important for understanding pathophysiological processes. However, existing fluorescent probes are unsuitable for these purposes due to their irreversible fluorogenic mechanisms or slow reaction rates. In this work, we have successfully overcome these problems by establishing a design strategy inspired by Mayr's work on nucleophilic reaction kinetics. The synthesized probes exhibit concentration-dependent, reversible and rapid absorption/fluorescence changes (t1/2 = 620 ms at [GSH] = 1 mM), as well as appropriate Kd values (1–10 mM: within the range of intracellular GSH concentrations). We also developed FRET-based ratiometric probes, and demonstrated that they are useful for quantifying GSH concentration in various cell types and also for real-time live-cell imaging of GSH dynamics with temporal resolution of seconds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
13 December 2016
In the version of this Article originally published, the y-axis of the middle panel in Fig. 1d was mislabelled. Molecular structures were also not present above Table 1. Both errors have been fixed in all versions of the Article.
References
Meister, A. & Anderson, M. E. Glutathione. Annu. Rev. Biochem. 52, 711–760 (1983).
Wu, G., Fang, Y.-Z., Yang, S., Lupton, J. R. & Turner, N. D. Glutathione metabolism and its implications for health. J. Nutr. 134, 489–492 (2004).
Townsend, D. M., Tew, K. D. & Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 57, 145–155 (2003).
Balendiran, G. K., Dabur, R. & Fraser, D. The role of glutathione in cancer. Cell Biochem. Fucnt. 22, 343–352 (2004).
Estrela, J. M., Ortega, A. & Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 43, 143–181 (2006).
Ishimoto, T. et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc− and thereby promotes tumor growth. Cancer Cell 19, 387–400 (2011).
Fierro, S. et al. In vivo assessment of cancerous tumors using boron doped diamond microelectrode. Sci. Rep. 2, 901 (2012).
Chen, X., Zhou, Y., Peng, X. & Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 39, 2120–2135 (2010).
Kim, G.-J., Lee, K., Kwon, H. & Kim, H.-J. Ratiometric fluorescence imaging of cellular glutathione. Org. Lett. 13, 2799–2801 (2011).
Niu, L.-Y. et al. BODIPY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 134, 18928–18931 (2012).
Yin, J. et al. Cyanine-based fluorescent probe for highly selective detection of glutathione in cell cultures and live mouse tissues. J. Am. Chem. Soc. 136, 5351–5358 (2014).
Lim, S. Y., Hong, K.-H., Kim, D. I., Kwon, H. & Kim, H.-J. Tunable heptamethine–azo dye conjugate as an NIR fluorescent probe for the selective detection of mitochondrial glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 136, 7018–7025 (2014).
Yoshida, M., Kamiya, M., Yamasoba, T. & Urano, Y. A highly sensitive, cell-membrane-permeable fluorescent probe for glutathione. Bioorg. Med. Chem. Lett. 24, 4363–4366 (2014).
Ahn, Y. H., Lee, J. S. & Chang, Y. T. Combinatorial rosamine library and application to in vivo glutathione probe. J. Am. Chem. Soc. 129, 4510–4511 (2007).
Xu, K. et al. A near-infrared reversible fluorescent probe for real-time imaging of redox status changes in vivo. Chem. Sci. 4, 1079–1086 (2013).
Chen, J., Jiang, X., Carroll, S. L., Huang, J. & Wang, J. Theoretical and experimental investigation of thermodynamics and kinetics of thiol-Michael addition reactions: a case study of reversible fluorescent probes for glutathione imaging in single cells. Org. Lett. 17, 5978–5981 (2015).
Jiang, X. et al. Quantitative imaging of glutathione in live cells using a reversible reaction-based ratiometric fluorescent probe. ACS Chem. Biol. 10, 864–874 (2015).
Kenmoku, S., Urano, Y., Kojima, H. & Nagano, T. Development of a highly specific rhodamine-based fluorescence probe for hypochlorous acid and its application to real-time imaging of phagocytosis. J. Am. Chem. Soc. 129, 7313–7318 (2007).
Sakabe, M. et al. Rational design of highly sensitive fluorescence probes for protease and glycosidase based on precisely controlled spirocyclization. J. Am. Chem. Soc. 135, 409–414 (2013).
Uno, S. et al. A spontaneously blinking fluorophore based on intramolecular spirocyclization for live-cell super-resolution imaging. Nat. Chem. 6, 681–689 (2014).
Mayr, H. Reactivity scales for quantifying polar organic reactivity: The benzhydrylium methodology. Tetrahedron 71, 5095–5111 (2015).
Appel, R. & Mayr, H. Quantification of the electrophilic reactivities of aldehydes, imines, and enones. J. Am. Chem. Soc. 133, 8240–8251 (2011).
Mayr, H. et al. Reference scales for the characterization of cationic electrophiles and neutral nucleophiles. J. Am. Chem. Soc. 123, 9500–9512 (2001).
Lavis, L. D. & Raines, R. T. Bright building blocks for chemical biology. ACS Chem. Biol. 9, 855–866 (2014).
Umezawa, K., Citterio, D. & Suzuki, K. New trends in near-infrared fluorophores for bioimaging. Anal. Sci. 30, 327–349 (2014).
Kushida, Y., Nagano, T. & Hanaoka, K. Silicon-substituted xanthene dyes and their applications to bioimaging. Analyst 140, 685–695 (2015).
Koide, Y., Urano, Y., Hanaoka, K., Terai, T. & Nagano, T. Evolution of group 14 rhodamines as platforms for near-infrared fluorescence probes utilizing photoinduced electron transfer. ACS Chem. Biol. 6, 600–608 (2011).
Lukinavičius, G. et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 5, 132–139 (2013).
Fu, M. Y., Xiao, Y., Qian, X. H., Zhao, D. F. & Xu, Y. F. A design concept of long-wavelength fluorescent analogs of rhodamine dyes: replacement of oxygen with silicon atom. Chem. Commun. 1780–1782 (2008).
Streidl, N., Denegri, B., Kronja, O. & Mayr, H. A practical guide for estimating rates of heterolysis reactions. Acc. Chem. Res. 43, 1537–1549 (2010).
Mayr, H. et al. Scales of Lewis basicities toward C-centered Lewis acids (carbocations). J. Am. Chem. Soc. 137, 2580–2599 (2015).
Marí, M., Morales, A., Colell, A., García-Ruiz, C. & Fernández-Checa, J. C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 11, 2685–2700 (2009).
Pompella, A., De Tata, V., Paolicchi, A. & Zunino, F. Expression of γ-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem. Pharmacol. 71, 231–238 (2006).
Urano, Y. et al. Rapid cancer detection by topically spraying a γ-glutamyltranspeptidase–activated fluorescent probe. Sci. Transl. Med. 3, 110ra119 (2011).
Hino, H. et al. Rapid cancer fluorescence imaging using a γ-glutamyltranspeptidase-specific probe for primary lung cancer. Transl. Oncol. 9, 203–210 (2016).
Anderson, M. E., Powrie, F., Puri, R. N. & Meister, A. Glutathione monoethyl ester: Preparation, uptake by tissues, and conversion to glutathione. Arch. Biochem. Biophys. 239, 538–548 (1985).
Schirmer, R. H., Müller, J. G. & Krauth-Siegel, R. L. Disulfide-reductase inhibitors as chemotherapeutic agents: the design of drugs for trypanosomiasis and malaria. Angew. Chem. Int. Ed. 34, 141–154 (1995).
Zhao, Y. et al. Increase in thiol oxidative stress via glutathione reductase inhibition as a novel approach to enhance cancer sensitivity to X-ray irradiation. Free Radical Biol. Med. 47, 176–183 (2009).
Ahmad, I. M. et al. Mitochondrial and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J. Biol. Chem. 280, 4254–4263 (2005).
Aykin-Burns, N., Ahmad, I. M., Zhu, Y., Oberley, L. W. & Spitz, D. R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem. J. 418, 29–37 (2009).
Seebacher, N. A., Richardson, D. R. & Jansson, P. J. Glucose modulation induces reactive oxygen species and increases P-glycoprotein-mediated multidrug resistance to chemotherapeutics. Br. J. Pharmacol. 172, 2557–2572 (2015).
Gutscher, M. et al. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 5, 553–559 (2008).
Nordberg, J. & Arnér, E. S. J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biol. Med. 31, 1287–1312 (2001).
Darby Weydert, C. J. et al. Inhibition of oral cancer cell growth by adenovirusMnSOD plus BCNU treatment. Free Radical Biol. Med. 34, 316–329 (2003).
Acknowledgements
This research was supported in part by AMED-CREST, by JST, PRESTO, by MEXT/JSPS KAKENHI grant numbers JP16H02606 and JP26111012 (to Y.U.), JP15H05951 ‘Resonance Bio’ (to M.K.), by JSPS Core-to-Core Program, by a grant from Hoansha Foundation (to Y.U.). The authors thank H. Takahashi for advices on intracellular imaging and statistical analysis, A. Morozumi for providing 2′Me SiR620, and Y. Kagami for advice on synthesis of Si-rhodamines.
Author information
Authors and Affiliations
Contributions
K.U., M.Y., M.K. and Y.U. designed the research. K.U. and M.Y. performed experiments and analysed the data. M.K., T.Y. and Y.U. supervised the project. All authors discussed the results and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3408 kb)
Supplementary movie
Supplementary movie 1 (AVI 2249 kb)
Supplementary movie
Supplementary movie 2 (AVI 2122 kb)
Supplementary movie
Supplementary movie 3 (AVI 2025 kb)
Supplementary movie
Supplementary movie 4 (AVI 1337 kb)
Supplementary movie
Supplementary movie 5 (AVI 1862 kb)
Rights and permissions
About this article
Cite this article
Umezawa, K., Yoshida, M., Kamiya, M. et al. Rational design of reversible fluorescent probes for live-cell imaging and quantification of fast glutathione dynamics. Nature Chem 9, 279–286 (2017). https://doi.org/10.1038/nchem.2648
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2648
This article is cited by
-
Controlled sequential in situ self-assembly and disassembly of a fluorogenic cisplatin prodrug for cancer theranostics
Nature Communications (2023)
-
A locally activatable sensor for robust quantification of organellar glutathione
Nature Chemistry (2023)
-
Designs and Applications of Multi-stimuli Responsive FRET Processes in AIEgen-Functionalized and Bi-fluorophoric Supramolecular Materials
Topics in Current Chemistry (2023)
-
Landomycins as glutathione-depleting agents and natural fluorescent probes for cellular Michael adduct-dependent quinone metabolism
Communications Chemistry (2021)
-
A general strategy to develop cell permeable and fluorogenic probes for multicolour nanoscopy
Nature Chemistry (2020)