Abstract
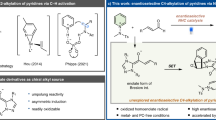
Saturated aza-heterocycles are highly privileged building blocks that are commonly encountered in bioactive compounds and approved therapeutic agents. These N-heterocycles are also incorporated as chiral auxiliaries and ligands in asymmetric synthesis. As such, the development of methods to functionalize the α-methylene C–H bonds of these systems enantioselectively is of great importance, especially in drug discovery. Currently, enantioselective lithiation with (–)-sparteine followed by Pd(0) catalysed cross-coupling to prepare α-arylated amines is largely limited to pyrrolidines. Here we report a Pd(II)-catalysed enantioselective α-C–H coupling of a wide range of amines, which include ethyl amines, azetidines, pyrrolidines, piperidines, azepanes, indolines and tetrahydroisoquinolines. Chiral phosphoric acids are demonstrated as effective anionic ligands for the enantioselective coupling of methylene C–H bonds with aryl boronic acids. This catalytic reaction not only affords high enantioselectivities, but also provides exclusive regioselectivity in the presence of two methylene groups in different steric environments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Royer, J. Asymmetric Synthesis of Nitrogen Heterocycles (Wiley-VCH, 2009).
Nugent, T. C. Chiral Amine Synthesis: Methods, Developments and Applications (Wiley-VCH, 2010).
Campos, K. R. Direct sp3 C–H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 36, 1069–1084 (2007).
Mitchell, E. A., Peschiulli, A., Lefevre, N., Meerpoel, L. & Maes, B. U. W. Direct α-functionalization of saturated cyclic amines. Chem. Eur. J. 18, 10092–10142 (2012).
Beak, P., Kerrick, S. T., Wu, S. & Chu, J. Complex induced proximity effects: enantioselective syntheses based on asymmetric deprotonations of N-Boc-pyrrolidines. J. Am. Chem. Soc. 116, 3231–3239 (1994).
Campos, K. R., Klapars, A., Waldman, J. H., Dormer, P. G. & Chen, C.-Y. Enantioselective, palladium-catalyzed α-arylation of N-Boc-pyrrolidine. J. Am. Chem. Soc. 128, 3538–3539 (2006).
Beng, T. K. & Gawley, R. E. Application of catalytic dynamic resolution of N-Boc-2-lithiopiperidine to the asymmetric synthesis of 2-aryl and 2-vinyl piperidines. Org. Lett. 13, 394–397 (2011).
Cordier, C. J., Lundgren, R. J. & Fu, G. C. Enantioconvergent cross-couplings of racemic alkylmetal reagents with unactivated secondary alkyl electrophiles: catalytic asymmetric Negishi α-alkylations of N-Boc-pyrrolidine. J. Am. Chem. Soc. 135, 10946–10949 (2013).
Stead, D. et al. Asymmetric deprotonation of N-Boc piperidine: react IR monitoring and mechanistic aspects. J. Am. Chem. Soc. 132, 7260–7261 (2010).
Spangler, J. E., Kobayashi, Y., Verma, P., Wang, D.-H. & Yu, J.-Q. α-Arylation of saturated azacycles and N-methylamines via palladium(II)-catalyzed C(sp3)−H coupling. J. Am. Chem. Soc. 137, 11876–11879 (2015).
Xiao, K.-J. et al. Palladium(II)-catalyzed enantioselective C(sp3)–H activation using a chiral hydroxamic acid ligand. J. Am. Chem. Soc. 136, 8138–8142 (2014).
Chan, K. S. L., Fu, H.-Y. & Yu, J.-Q. Palladium(II)-catalyzed highly enantioselective C–H arylation of cyclopropylmethylamines. J. Am. Chem. Soc. 136, 2042–2046 (2015).
Nakanishi, M., Katayev, D., Besnard, C. & Kündig, E. P. Fused indolines by palladium-catalyzed asymmetric C–C coupling involving an unactivated methylene group. Angew. Chem. Int. Ed. 50, 7438–7441 (2011).
Anas, S., Cordi, A. & Kagan, H. B. Enantioselective synthesis of 2-methyl indolines by palladium catalysed asymmetric C(sp3)–H activation/cyclisation. Chem. Commun. 47, 11483–11485 (2011).
Martin, N., Pierre, C., Davi, M., Jazzar, R. & Baudoin, O. Diastereo- and enantioselective intramolecular C(sp3)–H arylation for the synthesis of fused cyclopentanes. Chem. Eur. J. 18, 4480–4484 (2012).
Saget, T., Lemouzy, S. & Cramer, N. Chiral monodentate phosphines and bulky carboxylic acids: cooperative effects in palladium-catalyzed enantioselective C(sp3)–H functionalization. Angew. Chem. Int. Ed. 51, 2238–2242 (2012).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 43, 1566–1568 (2004).
Uraguchi, D. & Terada, M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 126, 5356–5357 (2004).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Hamilton, G. L., Kang, E. J., Mba, M. & Toste, F. D. A powerful chiral counterion strategy for asymmetric transition metal catalysis. Science 317, 496–499 (2007).
Mukherjee, S. & List, B. Chiral counteranions in asymmetric transition-metal catalysis: highly enantioselective Pd/Brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc. 129, 11336–11337 (2007).
Jiang, G., Halder, R., Fang, Y. & List, B. A highly enantioselective Overman rearrangement through asymmetric counteranion-directed palladium catalysis. Angew. Chem. Int. Ed. 50, 9752–9755 (2011).
Chai, Z. & Rainey, T. J. Pd(II)/Brønsted acid catalyzed enantioselective allylic C−H activation for the synthesis of spirocyclic rings. J. Am. Chem. Soc. 134, 3615–3618 (2012).
Wang, P.-S., Lin, H.-C., Zhai, Y.-J., Han, Z.-Y. & Gong, L.-Z. Chiral counteranion strategy for asymmetric oxidative C(sp3)–H/C(sp3)–H coupling: enantioselective α-allylation of aldehydes with terminal alkenes. Angew. Chem. Int. Ed. 53, 11218–11221 (2014).
Engle, K. M. & Yu, J.-Q. Developing ligands for palladium(II)-catalyzed C–H functionalization: intimate dialogue between ligand and substrate. J. Org. Chem. 78, 8927–8955 (2013).
Yan, S.-B., Zhang, S. & Duan, W.-L. Palladium-catalyzed asymmetric arylation of C(sp3)–H bonds of aliphatic amides: controlling enantioselectivity using chiral phosphoric amides/acids. Org. Lett. 17, 2458–2461 (2015).
Zhang, D. et al. Enantioselective palladium(II) phosphate catalyzed three-component reactions of pyrrole, diazoesters, and imines. Angew. Chem. Int. Ed. 52, 13356–13360 (2013).
Ding, J., Rybak, T. & Hall, D. G. Synthesis of chiral heterocycles by ligand-controlled regiodivergent and enantiospecific Suzuki–Miyaura cross-coupling. Nat. Commun. 5, 5474 (2014).
Bailey, W. F., Beak, P., Kerrick, S. T., Ma, S. & Wiberg, K. B. An experimental and computational investigation of the enantioselective deprotonation of Boc-piperidine. J. Am. Chem. Soc. 124, 1889–1896 (2002).
Hodgson, D. & Kloesges, J. Lithiation–electrophilic substitution of N-thiopivaloylazetidine. Angew. Chem. Int. Ed. 49, 2900–2903 (2010).
Hodgson, D., Mortimer, C. L. & McKenna, J. M. Amine protection/α-activation with the tert-butoxythiocarbonyl group: application to azetidine lithiation−electrophilic substitution. Org. Lett. 17, 330–333 (2015).
Li, X., Leonori, D., Sheikh, N. S. & Coldham, I. Synthesis of 1-substituted tetrahydroisoquinolines by lithiation and electrophilic quenching guided by in situ IR and NMR spectroscopy and application to the synthesis of salsolidine, carnegine and laudanosine. Chem. Eur. J. 19, 7724–7730 (2013).
Mitch, C. H. et al. Discovery of aminobenzyloxyarylamides as κ opioid receptor selective antagonists: application to preclinical development of a κ opioid receptor antagonist receptor occupancy tracer. J. Med. Chem. 54, 8000–8012 (2011).
Acknowledgements
We acknowledge The Scripps Research Institute and the National Institutes of Health (NIGMS, 2R01GM084019) for their financial support.
Author information
Authors and Affiliations
Contributions
P.J. developed the enantioselective arylation reaction. P.V. and G.X. expanded the substrate scope. J.-Q.Y. conceived and supervised the project. J.-Q.Y. and P.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 11312 kb)
Rights and permissions
About this article
Cite this article
Jain, P., Verma, P., Xia, G. et al. Enantioselective amine α-functionalization via palladium-catalysed C–H arylation of thioamides. Nature Chem 9, 140–144 (2017). https://doi.org/10.1038/nchem.2619
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2619
This article is cited by
-
Site- and enantioselective cross-coupling of saturated N-heterocycles with carboxylic acids by cooperative Ni/photoredox catalysis
Nature Communications (2023)
-
Transition metal-catalysed directed C–H functionalization with nucleophiles
Nature Synthesis (2022)
-
Copper catalyzed late-stage C(sp3)-H functionalization of nitrogen heterocycles
Nature Communications (2021)
-
Late-stage oxidative C(sp3)–H methylation
Nature (2020)
-
Expedited mapping of the ligandable proteome using fully functionalized enantiomeric probe pairs
Nature Chemistry (2019)