Abstract
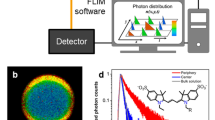
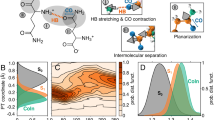
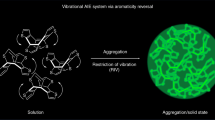
Although there are some proposed explanations for aggregation-induced emission, a phenomenon with applications that range from biosensors to organic light-emitting diodes, current understanding of the quantum-mechanical origin of this photophysical behaviour is limited. To address this issue, we assessed the emission properties of a series of BF2–hydrazone-based dyes as a function of solvent viscosity. These molecules turned out to be highly efficient fluorescent molecular rotors. This property, in addition to them being aggregation-induced emission luminogens, enabled us to probe deeper into their emission mechanism. Time-dependent density functional theory calculations and experimental results showed that the emission is not from the S1 state, as predicted from Kasha's rule, but from a higher energy (>S1) state. Furthermore, we found that suppression of internal conversion to the dark S1 state by restricting the rotor rotation enhances fluorescence, which leads to the proposal that suppression of Kasha's rule is the photophysical mechanism responsible for emission in both viscous solution and the solid state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Haidekker, M. A. & Theodorakis, E. A. Environment-sensitive behavior of fluorescent molecular rotors. J. Biol. Eng. 4, 11–24 (2010).
Kuimova, M. K. Mapping viscosity in cells using molecular rotors. Phys. Chem. Chem. Phys. 14, 12671–12686 (2012).
Srivatsan, S. G., Greco, N. J. & Tor, Y. A highly emissive fluorescent nucleoside that signals the activity of toxic ribosome-inactivating proteins. Angew. Chem. Int. Ed. 47, 6661–6665 (2008).
Kuimova, M. K. et al. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 1, 69–73 (2009).
Cao, K. et al. Aminonaphthalene 2-cyanoacrylate (ANCA) probes fluorescently discriminate between amyloid-β and prion plaques in brain. J. Am. Chem. Soc. 134, 17338–17341 (2012).
Wang, L., Xiao, Y., Tian, W. & Deng, L. Activatable rotor for quantifying lysosomal viscosity in living cells. J. Am. Chem. Soc. 135, 2903–2906 (2013).
Cui, M. C. et al. Smart near-infrared fluorescence probes with donor–acceptor structure for in vivo detection of β-amyloid deposits. J. Am. Chem. Soc. 136, 3388–3394 (2014).
Levitt, J. A. et al. Membrane-bound molecular rotors measure viscosity in live cells via fluorescence lifetime imaging. J. Phys. Chem. C 113, 11634–11642 (2009).
Hosny, N. A. et al. Mapping microbubble viscosity using fluorescence lifetime imaging of molecular rotors. Proc. Natl Acad. Sci. USA 110, 9225–9230 (2013).
Shiraishi, Y., Inoue, T. & Hirai, T. Local viscosity analysis of triblock copolymer micelle with cyanine dyes as a fluorescent probe. Langmuir 26, 17505–17512 (2010).
Vaccaro, G. et al. Direct monitoring of self-assembly of copolymeric micelles by a luminescent molecular rotor. Chem. Commun. 49, 8474–8476 (2013).
Feng, J. et al. A triarylboron-based fluorescent thermometer: sensitive over a wide temperature range. Angew. Chem. Int. Ed. 50, 8072–8076 (2011).
Peng, X. J. et al. Fluorescence ratiometry and fluorescence lifetime imaging: using a single molecular sensor for dual mode imaging of cellular viscosity. J. Am. Chem. Soc. 133, 6626–6635 (2011).
Gatzogiannis, E. et al. Mapping protein-specific micro-environments in live cells by fluorescence lifetime imaging of a hybrid genetic-chemical molecular rotor tag. Chem. Commun. 48, 8694–8696 (2012).
Haidekker, M. A., Brady, T. P., Lichlyter, D. & Theodorakis, E. A. Effects of solvent polarity and solvent viscosity on the fluorescent properties of molecular rotors and related probes. Bioorg. Chem. 33, 415–425 (2005).
Bochkov, A. Y., Akchurin, I. O., Dyachenko, O. A. & Traven, V. F. NIR-fluorescent coumarin-fused BODIPY dyes with large Stokes shifts. Chem. Commun. 49, 11653–11655 (2013).
Cosgrave, L., Devocelle, M., Forster, R. J. & Keyes, T. E. Multimodal cell imaging by ruthenium polypyridyl labelled cell penetrating peptides. Chem. Commun. 46, 103–105 (2010).
Zhou, Y., Xiao, Y., Chi, S. M. & Qian, X. H. Isomeric boron–fluorine complexes with donor–acceptor architecture: strong solid/liquid fluorescence and large Stokes shift. Org. Lett. 10, 633–636 (2008).
Yang, Y., Su, X., Carroll, C. N. & Aprahamian, I. Aggregation-induced emission in BF2-hydrazone (BODIHY) complexes. Chem. Sci. 3, 610–613 (2012).
Yang, Y. Hydrazone-Based Macrocycles, Fluorophores and Light-Activated Switches PhD Thesis, Dartmouth College (2014).
Su, X. & Aprahamian, I. Hydrazone-based switches, metallo-assemblies and sensors. Chem. Soc. Rev. 43, 1963–1981 (2014).
Tatum, L. A., Su, X. & Aprahamian, I. Simple hydrazone building blocks for complicated functional materials. Acc. Chem. Res. 47, 2141–2149 (2014).
Hong, Y. N., Lam, J. W. Y. & Tang, B. Z. Aggregation-induced emission. Chem. Soc. Rev. 40, 5361–5388 (2011).
Leung, N. L. C. et al. Restriction of intramolecular motions: the general mechanism behind aggregation-induced emission. Chem. Eur. J. 20, 15349–15353 (2014).
Mei, J., Leung, N. L. C., Kwok, R. T. K., Lam, J. W. Y. & Tang, B. Z. Aggregation-induced emission: together we shine, united we soar! Chem. Rev. 115, 11718–11940 (2015).
Born, M. & Oppenheimer, R. Zur Quantentheorie der Molekeln [in German]. Ann. Phys. 389, 457–484 (1927).
Kee, H. L. et al. Structural control of the photodynamics of boron–dipyrrin complexes. J. Phys. Chem. B 109, 20433–20443 (2005).
Zhou, F. K. et al. Molecular rotors as fluorescent viscosity sensors: molecular design, polarity sensitivity, dipole moments changes, screening solvents, and deactivation channel of the excited states. Eur. J. Org. Chem. 4773–4787 (2011).
Shao, J. Y. et al. Thiophene-inserted aryl-dicyanovinyl compounds: the second generation of fluorescent molecular rotors with significantly redshifted emission and large Stokes shift. Eur. J. Org. Chem. 6100–6109 (2011).
Peng, Q., Niu, Y. L., Deng, C. M. & Shuai, Z. G. Vibration correlation function formalism of radiative and non-radiative rates for complex molecules. Chem. Phys. 370, 215–222 (2010).
Shuai, Z. G. & Peng, Q. Excited states structure and processes: understanding organic light-emitting diodes at the molecular level. Phys. Rep. 537, 123–156 (2014).
Kasha, M. Characterization of electronic transitions in complex molecules. Disc. Faraday Soc. 14–19 (1950).
Munkholm, C., Parkinson, D. R. & Walt, D. R. Intramolecular fluorescence self-quenching of fluoresceinamine. J. Am. Chem. Soc. 112, 2608–2612 (1990).
Ueno, T., Urano, Y., Kojima, H. & Nagano, T. Mechanism-based molecular design of highly selective fluorescence probes for nitrative stress. J. Am. Chem. Soc. 128, 10640–10641 (2006).
Jacquemin, D., Mennucci, B. & Adamo, C. Excited-state calculations with TD-DFT: from benchmarks to simulations in complex environments. Phys. Chem. Chem. Phys. 13, 16987–16998 (2011).
Englman, R. The energy gap law for radiationless transitions in large molecules. Mol. Phys. 18, 145–164 (1970).
Itoh, T. Fluorescence and phosphorescence from higher excited states of organic molecules. Chem. Rev. 112, 4541–4568 (2012).
Mei, J. et al. Aggregation-induced emission: the whole is more brilliant than the parts. Adv. Mater. 26, 5429–5479 (2014).
Acknowledgements
I.A. and M.D.L. acknowledge the support of the National Science Foundation (DMR-1506170 and DMR-1506248, respectively). A.W. acknowledges the support of the ACS Project SEED program.
Author information
Authors and Affiliations
Contributions
All the experiments were conducted by H.Q., M.E.C., E.H.H. and A.W. with input from I.A. and M.D.L. All the computational work was conducted by M.E.C. and E.H.H. with the supervision of M.D.L. The manuscript was written jointly by H.Q., I.A., M.E.C. and M.D.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3974 kb)
Supplementary information
Crystallographic data for compound 2. (CIF 242 kb)
Supplementary information
Structure factors file for compound 2. (FCF 132 kb)
Rights and permissions
About this article
Cite this article
Qian, H., Cousins, M., Horak, E. et al. Suppression of Kasha's rule as a mechanism for fluorescent molecular rotors and aggregation-induced emission. Nature Chem 9, 83–87 (2017). https://doi.org/10.1038/nchem.2612
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2612
This article is cited by
-
Machine-learning screening of luminogens with aggregation-induced emission characteristics for fluorescence imaging
Journal of Nanobiotechnology (2023)
-
Understanding H-aggregates crystallization induced emissive behavior: insights from theory
Scientific Reports (2023)
-
A platform for blue-luminescent carbon-centered radicals
Nature Communications (2022)
-
Thiophenitrile triphenylamine as a viscosity-sensitive molecular rotor toward liquid safety inspection
Journal of Food Measurement and Characterization (2022)
-
Unexpected organic hydrate luminogens in the solid state
Nature Communications (2021)