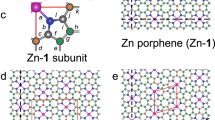
Abstract
Surface-assisted covalent linking of precursor molecules enables the fabrication of low-dimensional nanostructures, which include graphene nanoribbons. One approach to building functional multicomponent systems involves the lateral anchoring of organic heteromolecules to graphene. Here we demonstrate the dehydrogenative coupling of single porphines to graphene edges on the same metal substrate as used for graphene synthesis. The covalent linkages are visualized by scanning probe techniques with submolecular resolution, which directly reveals bonding motifs and electronic features. Distinct configurations are identified that can be steered towards entities predominantly fused to graphene edges through two pyrrole rings by thermal annealing. Furthermore, we succeeded in the concomitant metallation of the macrocycle with substrate atoms and the axial ligation of adducts. Such processes combined with graphene–nanostructure synthesis has the potential to create complex materials systems with tunable functionalities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Méndez, J., López, M. F. & Martín-Gago, J. A. On-surface synthesis of cyclic organic molecules. Chem. Soc. Rev. 40, 4578–4590 (2011).
Lafferentz, L. et al. Controlling on-surface polymerization by hierarchical and substrate-directed growth. Nat. Chem. 4, 215–220 (2012).
Chen, Y.-C. et al. Molecular bandgap engineering of bottom-up synthesized graphene nanoribbon heterojunctions. Nat. Nanotech. 10, 156–160 (2015).
Dyke, C. A. & Tour, J. M. Covalent functionalization of single-walled carbon nanotubes for materials applications. J. Phys. Chem. A 108, 11151–11159 (2004).
Haq, S. et al. Versatile bottom-up construction of diverse macromolecules on a surface observed by scanning tunneling microscopy. ACS Nano 8, 8856–8870 (2014).
Gottfried, J. M. Surface chemistry of porphyrins and phthalocyanines. Surf. Sci. Rep. 70, 259–379 (2015).
Auwärter, W., Écija, D., Klappenberger, F. & Barth, J. V. Porphyrins at interfaces. Nat. Chem. 7, 105–120 (2015).
Auwärter, W. et al. A surface-anchored molecular four-level conductance switch based on single proton transfer. Nat. Nanotech. 7, 41–46 (2011).
Seufert, K. et al. Cis-dicarbonyl binding at cobalt and iron porphyrins with saddle-shape conformation. Nat. Chem. 3, 114–119 (2011).
Drain, C. M., Varotto, A. & Radivojevic, I. Self-organized porphyrinic materials. Chem. Rev. 109, 1630–1658 (2009).
Urbani, M., Gratzel, M., Nazeeruddin, M. K. & Torres, T. Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 114, 12330–12396 (2014).
Mathew, S. et al. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 6, 242–247 (2014).
Neto, A. C., Guinea, F., Peres, N., Novoselov, K. S. & Geim, A. K. The electronic properties of graphene. Rev. Mod. Phys. 81, 109–162 (2009).
Georgakilas, V. et al. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 112, 6156–6214 (2012).
Malig, J., Jux, N. & Guldi, D. M. Toward multifunctional wet chemically functionalized graphene—integration of oligomeric, molecular, and particulate building blocks that reveal photoactivity and redox activity. Acc. Chem. Res. 46, 53–64 (2012).
Dai, L. Functionalization of graphene for efficient energy conversion and storage. Acc. Chem. Res. 46, 31–42 (2012).
Criado, A., Melchionna, M., Marchesan, S. & Prato, M. The covalent functionalization of graphene on substrates. Angew. Chem. Int. Ed. 54, 10734–10750 (2015).
Kuila, T. et al. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 57, 1061–1105 (2012).
Xu, Y. et al. A graphene hybrid material covalently functionalized with porphyrin: synthesis and optical limiting property. Adv. Mater. 21, 1275–1279 (2009).
Roy-Mayhew, J. D. & Aksay, I. A. Graphene materials and their use in dye-sensitized solar cells. Chem. Rev. 114, 6323–6348 (2014).
Bae, S. et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotech. 5, 574–578 (2010).
Cai, J. et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466, 470–473 (2010).
Otero, G. et al. Fullerenes from aromatic precursors by surface-catalysed cyclodehydrogenation. Nature 454, 865–868 (2008).
Cai, J. et al. Graphene nanoribbon heterojunctions. Nat. Nanotech. 9, 896–900 (2014).
Kawai, S. et al. Atomically controlled substitutional boron-doping of graphene nanoribbons. Nat. Commun. 6, 8098 (2015).
Treier, M. et al. Surface-assisted cyclodehydrogenation provides a synthetic route towards easily processable and chemically tailored nanographenes. Nat. Chem. 3, 61–67 (2011).
Fan, Q., Gottfried, J. M. & Zhu, J. Surface-catalyzed C–C covalent coupling strategies toward the synthesis of low-dimensional carbon-based nanostructures. Acc. Chem. Res. 48, 2484–2494 (2015).
Riss, A. et al. Local electronic and chemical structure of oligo-acetylene derivatives formed through radical cyclizations at a surface. Nano Lett. 14, 2251–2255 (2014).
Nacci, C. et al. Conductance of a single flexible molecular wire composed of alternating donor and acceptor units. Nat. Commun. 6, 7397 (2015).
Klappenberger, F. et al. On-surface synthesis of carbon-based scaffolds and nanomaterials using terminal alkynes. Acc. Chem. Res. 48, 2140–2150 (2015).
Deng, D. et al. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotech. 11, 218–230 (2015).
Wiengarten, A. et al. Surface-assisted dehydrogenative homocoupling of porphine molecules. J. Am. Chem. Soc. 136, 9346–9354 (2014).
Kiraly, B. et al. Solid-source growth and atomic-scale characterization of graphene on Ag(111). Nat. Commun. 4, 2804 (2013).
Xu, B. et al. Electronic and magnetic properties of zigzag graphene nanoribbon with one edge saturated. Appl. Phys. Lett. 96, 163102 (2010).
Batzill, M. The surface science of graphene: metal interfaces, CVD synthesis, nanoribbons, chemical modifications, and defects. Surf. Sci. Rep. 67, 83–115 (2012).
Bischoff, F. et al. How surface bonding and repulsive interactions cause phase transformations: ordering of a prototype macrocyclic compound on Ag(111). ACS Nano 7, 3139–3149 (2013).
Leicht, P. et al. In situ fabrication of quasi-free-standing epitaxial graphene nanoflakes on gold. ACS Nano 8, 3735–3742 (2014).
Merino, P. et al. Sublattice localized electronic states in atomically resolved graphene–Pt(111) edge-boundaries. ACS Nano 8, 3590–3596 (2014).
Garcia-Lekue, A. et al. Substrate-induced stabilization and reconstruction of zigzag edges in graphene nanoislands on Ni(111). J. Phys. Chem. C 119, 4072–4078 (2015).
de Oteyza, D. G. et al. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science 340, 1434–1437 (2013).
Dienel, T. et al. Resolving atomic connectivity in graphene nanostructure junctions. Nano Lett. 15, 5185–5190 (2015).
Schuler, B. et al. From perylene to a 22-ring aromatic hydrocarbon in one-pot. Angew. Chem. Int. Ed. 53, 9004–9006 (2014).
Albrecht, F., Pavliček, N., Herranz-Lancho, C., Ruben, M. & Repp, J. Characterization of a surface reaction by means of atomic force microscopy. J. Am. Chem. Soc. 137, 7424–7428 (2015).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Mohn, F., Gross, L., Moll, N. & Meyer, G. Imaging the charge distribution within a single molecule. Nat. Nanotech. 7, 227–231 (2012).
Neu, M. et al. Image correction for atomic force microscopy images with functionalized tips. Phys. Rev. B 89, 205407 (2014).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Chuvilin, A., Meyer, J. C., Algara-Siller, G. & Kaiser, U. From graphene constrictions to single carbon chains. New J. Phys. 11, 083019 (2009).
Jin, C., Lan, H., Peng, L., Suenaga, K. & Iijima, S. Deriving carbon atomic chains from graphene. Phys. Rev. Lett. 102, 205501 (2009).
Chen, B. M. & Tulinsky, A. Redetermination of the structure of porphine. J. Am. Chem. Soc. 94, 4144–4151 (1972).
Marbach, H. Surface-mediated in situ metalation of porphyrins at the solid–vacuum interface. Acc. Chem. Res. 48, 2649–2658 (2015).
Diller, K. et al. In-vacuo interfacial tetrapyrrole metallation. Chem. Soc. Rev. 45, 1629–1656 (2016).
Wäckerlin, C. et al. Controlling spins in adsorbed molecules by a chemical switch. Nat. Commun. 1, 61 (2010).
Wäckerlin, C. et al. On-surface coordination chemistry of planar molecular spin systems: novel magnetochemical effects induced by axial ligands. Chem. Sci. 3, 3154–3160 (2012).
den Boer, D. et al. Detection of different oxidation states of individual manganese porphyrins during their reaction with oxygen at a solid/liquid interface. Nat. Chem. 5, 621–627 (2013).
Smykalla, L., Shukrynau, P., Zahn, D. R. & Hietschold, M. Self-metalation of phthalocyanine molecules with silver surface atoms by adsorption on Ag(110). J. Phys. Chem. C 119, 17228–17234 (2015).
Seufert, K., Auwärter, W. & Barth, J. V. Discriminative response of surface-confined metalloporphyrin molecules to carbon and nitrogen monoxide. J. Am. Chem. Soc. 132, 18141–18146 (2010).
Ijäs, M. et al. Electronic states in finite graphene nanoribbons: effect of charging and defects. Phys. Rev. B 88, 075429 (2013).
van der Lit, J. et al. Suppression of electron–vibron coupling in graphene nanoribbons contacted via a single atom. Nat. Commun. 4, 2023 (2013).
Tripkovic, V. et al. Electrochemical CO2 and CO reduction on metal-functionalized porphyrin-like graphene. J. Phys. Chem. C 117, 9187–9195 (2013).
Nečas, D. & Klapetek, P. Gwyddion: an open-source software for SPM data analysis. Open Phys. 10, 181–188 (2012).
Acknowledgements
The authors thank A. Riss for fruitful discussions. We are grateful for support from the European Research Council (ERC) Advanced Grant MolArt (no. 247299), the Munich Centre for Advanced Photonics and the TUM Institute for Advanced Study funded by the German Research Foundation (DFG) via the German Excellence Initiative as well as the European Union Seventh Framework Programme under grant agreement no. 291763. W.A. acknowledges funding from the DFG via a Heisenberg professorship and from the ERC Consolidator Grant NanoSurfs (no. 615233). M.B. acknowledges support from the National Science Foundation under award DMR-1204924. M.G. acknowledges financial support from the Marie Curie Intra-European Fellowship (Project 2D Nano, no. 658070).
Author information
Authors and Affiliations
Contributions
W.A. and J.V.B. conceived and designed the experiments. Y.H., F.B., J.D. and M.G. performed the experiments. Y.H., F.B., J.D. and M.G. analysed the data. M.-L.B. performed the calculations. M.G., W.A., Y.H., M.B. and J.V.B. co-wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 896 kb)
Rights and permissions
About this article
Cite this article
He, Y., Garnica, M., Bischoff, F. et al. Fusing tetrapyrroles to graphene edges by surface-assisted covalent coupling. Nature Chem 9, 33–38 (2017). https://doi.org/10.1038/nchem.2600
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2600
This article is cited by
-
Porphyrin-fused graphene nanoribbons
Nature Chemistry (2024)
-
Universal inter-molecular radical transfer reactions on metal surfaces
Nature Communications (2024)
-
Dramatic femtosecond nonlinear absorption at a strongly coupled porphyrin-graphene nanoconjugate
Nano Research (2023)
-
Atomically resolved TEM imaging of covalently functionalised graphene
npj 2D Materials and Applications (2022)
-
Long-range ordered and atomic-scale control of graphene hybridization by photocycloaddition
Nature Chemistry (2020)