Abstract
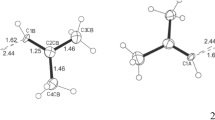
Vinylidene (H2C=C) is a member of the family of compounds of composition CH (and isomeric with ethyne, HC≡CH), but it has been observed only transiently—with a lifetime in the region of 0.1 ns. Indeed, no simple (non-base-stabilized) compounds of the type R2E=E have been characterized structurally for any of the group 14 elements. Here we show that by employing the bulky and strongly electron-donating boryl ligand (HCDippN)2B (Dipp, 2,6-iPr2C6H3), a simple monomeric digermavinylidene compound, (boryl)2GeGe, can be synthesized and is stable at room temperature. Both its formation via the two-electron chemical oxidation of the symmetrical Ge0 compound K2[(boryl)GeGe(boryl)] and its subsequent reaction chemistry (for example, with H2), are consistent with a high substituent lability and the accessibility of both 1,1- and 1,2-substitution patterns. Structural and computational studies of [(HCDippN)2B]2GeGe reveal a weak Ge–Ge double bond—the π component of which contributes to the highest occupied molecular orbital (HOMO)—with a Ge-centred lone pair as the HOMO–1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
DeKock, R. L. & Gray, H. B. Chemical Structure and Bonding (Univ. Science Books, 1989).
Simonetta, M. & Gavezzotti, A. in The Chemistry of the Carbon–Carbon Triple Bond (ed. Patai, S.) (Wiley, 1978).
Kekulé, A. Sur la constitution des substances aromatiques. Bull. Soc. Chim. Fr. 3, 98–110 (1865).
Cava, M. P. & Mitchell, M. J. Cyclobutadiene and Related Compounds (Academic, 1967).
Eaton, P. E. & Cole, T. W. Cubane. J. Am. Chem. Soc. 86, 3157–3158 (1964).
Fischer, R. C. & Power, P. P. π-Bonding and the lone pair effect in multiple bonds involving heavier main group elements—developments in the new millennium. Chem. Rev. 110, 3877–3923 (2010).
Power, P. P. Bonding and reactivity of heavier group 14 element alkyne analogues. Organometallics 26, 4362–4372 (2007).
Lee, V. Y. & Sekiguchi, A. Aromaticity of group 14 organometallics: experimental aspects. Angew. Chem. Int. Ed. 46, 6596–6620 (2007).
Abersfelder, K., White, A. J. P., Rzepa, H. S. & Scheschkewitz, D. A tricyclic aromatic isomer of hexasilabenzene. Science 327, 564–566 (2010).
Power, P. P. Main-group elements as transition metals. Nature 463, 171–177 (2010).
Stang, P. J. Unsaturated carbenes. Chem. Rev. 78, 383–405 (1978).
Bruce, M. I. Organometallic chemistry of vinylidene and related unsaturated carbenes. Chem. Rev. 91, 197–257 (1991).
Ervin, K. M. et al. Bond strengths of ethylene and acetylene. J. Am. Chem. Soc. 112, 5750–5759 (1990).
Krishnan, R., Frisch, M. J., Pople, J. A. & Schleyer, P. v. R. The vinylidene–acetylene isomerization barrier. Chem. Phys. Lett. 79, 408–411 (1981).
Osamura, Y. Schaefer, H. F. III, Gray, S. K. & Miller, W. H. Vinylidene: a very shallow minimum on the C2H2 potential energy surface. J. Am. Chem. Soc. 103, 1904–1907 (1981).
Chang, N.-Y. Shen, M.-Y. & Yu, C.-H. Extended ab initio studies of the vinylidene–acetylene rearrangement. J. Chem. Phys. 106, 3237–3242 (1997).
Stanton, J. F. & Gauss, J. Some predictions relevant to future spectroscopic observation of S1 vinylidene. J. Chem. Phys. 101, 3001–3005 (1994).
Breidung, J. et al. Difluorovinylidene, F2C=C. Angew. Chem. Int. Ed. Engl. 36, 1983–1985 (1997).
Brody, H. K., Magers, D. H. & Leszczyński, J. Ab initio studies of methylenecarbene and isoelectronic species. Struct. Chem. 6, 293–300 (1995).
Nagase, S., Kobayashi, K. & Takagi, N. Triple bonds between heavier group 14 elements. A theoretical approach. J. Organomet. Chem. 611, 264–271 (2000).
Lein, M., Krapp, A. & Frenking, G. Why do the heavy-atom analogues of acetylene E2H2 (E = Si−Pb) exhibit unusual structures? J. Am. Chem. Soc. 127, 6290–6299 (2005).
Landis, C. R. & Weinhold, F. Origin of trans-bent geometries in maximally bonded transition metal and main group molecules. J. Am. Chem. Soc. 128, 7335–7345 (2006).
Rivard, E. Group 14 inorganic hydrocarbon analogues. Chem. Soc. Rev. 45, 989–1003 (2016).
Harper, W. W. et al. Laser spectroscopic detection of the simplest unsaturated silylene and germylene. J. Am. Chem. Soc. 119, 8361–8362 (1997).
Chardonnier, M., Bogey, M., Demuynck, C. & Destombes, J.-L. Nonclassical structures in silicon-containing molecules: the monobridged isomer of Si2H2 . J. Chem. Phys. 97, 7984–7989 (1992).
Pu, L., Twamley, B. & Power, P. P. Synthesis and characterization of 2,6-Trip2H3C6PbPbC6H3-2,6-Trip2 (Trip = C6H2-2,4,6-i-Pr3): a stable heavier group 14 element analogue of an alkyne. J. Am. Chem. Soc. 122, 3524–3525 (2000).
Phillips, A. D., Wright, R. J., Olmstead, M. M. & Power, P. P. Synthesis and characterization of 2,6-Dipp2-H3C6SnSnC6H3-2,6-Dipp2 (Dipp = C6H3-2,6-iPr2): a tin analogue of an alkyne. J. Am. Chem. Soc. 124, 5930–5931 (2002).
Stender, M., Phillips, A. D., Wright, R. J. & Power, P. P. Synthesis and characterization of a digermanium analogue of an alkyne. Angew. Chem. Int. Ed. 41, 1785–1787 (2002).
Sekiguchi, A., Kinjo, R. & Ichinohe, M. A stable compound containing a silicon–silicon triple bond. Science 305, 1755–1757 (2004).
Jana, A., Huch, V. & Scheschkewitz, D. NHC-stabilized silagermenylidene: a heavier analogue of vinylidene. Angew. Chem. Int. Ed. 52, 12179–12182 (2013).
Jana, A., Majumdar, M., Huch, V., Zimmer, M. & Scheschkewitz, D. NHC-coordinated silagermenylidene functionalized in allylic position and its behaviour as a ligand. Dalton Trans. 43, 5175–5181 (2014).
Ghana, P., Arz, M. I., Das, U., Schnakenburg, G. & Filippou, A. C. Si=Si double bonds: synthesis of an NHC-stabilized disilavinylidene. Angew. Chem. Int. Ed. 54, 9980–9985 (2015).
Leung, W. P., Chan, Y.-C. & So, C.-W. Chemistry of heavier group 14 base-stabilized heterovinylidenes. Organometallics 34, 2067–2085 (2015).
Spikes, G. H., Fettinger, J. C. & Power, P. P. Facile activation of dihydrogen by an unsaturated heavier main group compound. J. Am. Chem. Soc. 127, 12232–12233 (2005).
Fischer, R. C., Pu, L., Fettinger, J. C., Brynda, M. A. & Power, P. P. Very large changes in bond length and bond angle in a heavy group 14 element alkyne analogue by modification of a remote ligand substituent. J. Am. Chem. Soc. 128, 11366–11367 (2006).
Sugiyama, Y. et al. Synthesis and properties of a new kinetically stabilized digermyne: new insights for a germanium analogue of an alkyne. J. Am. Chem. Soc. 128, 1023–1031 (2006).
Li, J., Schenk, C., Goedecke, C., Frenking, G. & Jones, C. A digermyne with a Ge–Ge single bond that activates dihydrogen in the solid state. J. Am. Chem. Soc. 133, 18622–18625 (2011).
Hadlington, T. J., Hermann, M., Li, J., Frenking, G. & Jones, C. Activation of H2 by a multiply bonded amido-digermyne: evidence for the formation of a hydrido-germylene. Angew. Chem. Int. Ed. 52, 10199–10203 (2013).
Sasamori, T. et al. Synthesis and reactions of a stable 1,2-diaryl-1,2-dibromodisilene: a precursor for substituted disilenes and a 1,2-diaryldisilyne. J. Am. Chem. Soc. 130, 13856–13857 (2008).
Protchenko, A. V. et al. A stable two-coordinate acyclic silylene. J. Am. Chem. Soc. 134, 6500–6503 (2012).
Protchenko, A. V. et al. Stable GaX2, InX2 and TlX2 radicals. Nat. Chem. 6, 315–319 (2014).
Braunschweig, H., Dewhurst, R. D. & Schneider, A. Electron-precise coordination modes of boron-centered ligands. Chem. Rev. 110, 3924–3957 (2010).
Segawa, Y., Yamashita, M. & Nozaki, K. Boryllithium: isolation, characterization, and reactivity as a boryl anion. Science 314, 113–115 (2006).
Rupar, P. A., Staroverov, V. N., Ragogna, P. J. & Baines, K. M. A germanium(II)-centered dication. J. Am. Chem. Soc. 129, 15138–15139 (2007).
Green, S. P., Jones, C. & Stasch, A. Stable magnesium(I) compounds with Mg–Mg bonds. Science 318, 1754–1757 (2007).
Pu, L. et al. Germanium and tin analogues of alkynes and their reduction products. J. Am. Chem. Soc. 125, 11626–11636 (2003).
Cui, C., Olmstead, M. M., Fettinger, J. C., Spikes, G. H. & Power, P. P. Reactions of the heavier group 14 element alkyne analogues ArʹEEArʹ (Arʹ = C6H3-2,6(C6H3-2,6-iPr2)2; E = Ge, Sn) with unsaturated molecules: probing the character of the EE multiple bonds. J. Am. Chem. Soc. 127, 17530–17541 (2005).
Broeckaert, L., Geerlings, P., Růžička, A., Willem, R. & De Proft, F. Can aromatic π-clouds complex divalent germanium and tin compounds? A DFT study. Organometallics 31, 1605–1617 (2012).
Takagi, N. & Nagase, S. Substituent effects on germanium−germanium and tin−tin triple bonds. Organometallics 20, 5498–5500 (2001).
Kostić, N. & Fenske, R. F. Molecular orbital study of bonding, conformations, and reactivity of transition-metal complexes containing unsaturated organic ligands. Electrophilic and nucleophilic additions to acetylide, vinylidene, vinyl, and carbene ligands. Organometallics 1, 974–982 (1982).
Acknowledgements
This work was supported by the Marie Curie Intra-European Fellowships programme of the European Union (PIEF-GA-2013-622806) and the Engineering and Physical Sciences Research Council (EP/L025000/1).
Author information
Authors and Affiliations
Contributions
A.R. synthesized and characterized the compounds. A.R. and J.C. collected the single-crystal X-ray crystallographic data and solved the crystal structures. A.R. and H.N. carried out the DFT calculations. S.A. generated and managed the project and wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1046 kb)
Supplementary information
Crystallographic data for compound 1 (CIF 34 kb)
Supplementary information
Structure factors file for compound 1 (FCF 547 kb)
Supplementary information
Crystallographic data for compound 2 (CIF 8685 kb)
Supplementary information
Crystallographic data for compound 3.1/2(C7H8) (CIF 1658 kb)
Supplementary information
Crystallographic data for compound 4 (CIF 1630 kb)
Supplementary information
Crystallographic data for compound 5 (CIF 1137 kb)
Supplementary information
Crystallographic data for compound 6 (CIF 643 kb)
Rights and permissions
About this article
Cite this article
Rit, A., Campos, J., Niu, H. et al. A stable heavier group 14 analogue of vinylidene. Nature Chem 8, 1022–1026 (2016). https://doi.org/10.1038/nchem.2597
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2597
This article is cited by
-
A silylene-stabilized ditin(0) complex and its conversion to methylditin cation and distannavinylidene
Nature Communications (2023)
-
A silylene-stabilized distannavinylidene with a highly labile substituent
Science China Chemistry (2023)
-
A sandwich-type cluster containing Ge@Pd3 planar fragment flanked by aromatic nonagermanide caps
Nature Communications (2020)
-
Reactivity of silagermenylidene toward nitrous oxide: a preliminary DFT study
Journal of Molecular Modeling (2018)
-
Dimeric magnesium(I) β-diketiminates: a new class of quasi-universal reducing agent
Nature Reviews Chemistry (2017)