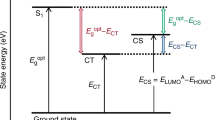
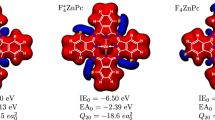
Abstract
Molecular approaches to solar-energy conversion require a kinetic optimization of light-induced electron-transfer reactions. At molecular–semiconductor interfaces, this optimization has previously been accomplished through control of the distance between the semiconductor donor and the molecular acceptor and/or the free energy that accompanies electron transfer. Here we show that a kinetic pathway for electron transfer from a semiconductor to a molecular acceptor also exists and provides an alternative method for the control of interfacial kinetics. The pathway was identified by the rational design of molecules in which the distance and the driving force were held near parity and only the geometric torsion about a xylyl- or phenylthiophene bridge was varied. Electronic coupling through the phenyl bridge was a factor of ten greater than that through the xylyl bridge. Comparative studies revealed a significant bridge dependence for electron transfer that could not be rationalized by a change in distance or driving force. Instead, the data indicate an interfacial electron-transfer pathway that utilizes the aromatic bridge orbitals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Closs, G. L. & Miller, J. R. Intramolecular long-distance electron transfer in organic molecules. Science 240, 440–447 (1988).
Davis, W. B., Svec, W. A., Ratner, M. A. & Wasielewski, M. R. Molecular-wire behaviour in p-phenylenevinylene oligomers. Nature 396, 60–63 (1998).
Lambert, C., Noll, G. & Schelter, J. Bridge-mediated hopping or superexchange electron-transfer processes in bis(triarylamine) systems. Nature Mater. 1, 69–73 (2002).
Vura-Weis, J. et al. Crossover from single-step tunneling to multistep hopping for molecular triplet energy transfer. Science 328, 1547–1550 (2010).
Ardo, S. & Meyer, G. J. Photodriven heterogeneous charge transfer with transition-metal compounds anchored to TiO2 semiconductor surfaces. Chem. Soc. Rev. 38, 115–164 (2009).
Abrahamsson, M. et al. Decreased interfacial charge recombination rate constants with N3-type sensitizers. J. Phys. Chem. Lett. 1, 1725–1728 (2010).
Asbury, J. B., Hao, E. C., Wang, Y. Q. & Lian, T. Q. Bridge length-dependent ultrafast electron transfer from Re polypyridyl complexes to nanocrystalline TiO2 thin films studied by femtosecond infrared spectroscopy. J. Phys. Chem. B 104, 11957–11964 (2000).
Haque, S. A. et al. Supermolecular control of charge transfer in dye-sensitized nanocrystalline TiO2 films: towards a quantitative structure–function relationship. Angew. Chem. Int. Ed. 44, 5740–5744 (2005).
Cameron, P. J. & Peter, L. M. Characterization of titanium dioxide blocking layers in dye-sensitized nanocrystalline solar cells. J. Phys. Chem. B 107, 14394–14400 (2003).
Palomares, E., Clifford, J. N., Haque, S. A., Lutz, T. & Durrant, J. R. Control of charge recombination dynamics in dye sensitized solar cells by the use of conformally deposited metal oxide blocking layers. J. Am. Chem. Soc. 125, 475–482 (2003).
Taube, H. & Myers, H. Evidence for a bridged activated complex for electron transfer reactions. J. Am. Chem. Soc. 76, 2103–2111 (1954).
Beratan, D. N., Betts, J. N. & Onuchic, J. N. Protein electron transfer rates set by the bridging secondary and tertiary structure. Science 252, 1285–1288 (1991).
Beratan, D. N., Onuchic, J. N., Winkler, J. R. & Gray, H. B. Electron-tunneling pathways in proteins. Science 258, 1740–1741 (1992).
Wenger, O. S. Photoinduced electron and energy transfer in phenylene oligomers. Chem. Soc. Rev. 40, 3538–3550 (2011).
Hopfield, J. J. Electron transfer between biological molecules by thermally activated tunneling. Proc. Natl Acad. Sci. USA 71, 3640–3644 (1974).
Moser, C., Page, C., Farid, R. & Dutton, P. L. Biological electron transfer. J. Bioenerg. Biomembr. 27, 263–274 (1995).
Haque, S. A., Tachibana, Y., Klug, D. R. & Durrant, J. R. Charge recombination kinetics in dye-sensitized nanocrystalline titanium dioxide films under externally applied bias. J. Phys. Chem. B 102, 1745–1749 (1998).
Brigham, E. C. & Meyer, G. J. Ostwald isolation to determine the reaction order for TiO2(e–)|S+ → TiO2|S charge recombination at sensitized TiO2 interfaces. J. Phys. Chem. C 118, 7886–7893 (2014).
Clifford, J. N. et al. Molecular control of recombination dynamics in dye-sensitized nanocrystalline TiO2 films: free energy vs distance dependence. J. Am. Chem. Soc. 126, 5225–5233 (2004).
Hasselmann, G. M. & Meyer, G. J. Diffusion-limited interfacial electron transfer with large apparent driving forces. J. Phys. Chem. B 103, 7671–7675 (1999).
Maggio, E. & Troisi, A. Theory of the charge recombination reaction at the semiconductor–adsorbate interface in the presence of defects. J. Phys. Chem. C 117, 24196–24205 (2013).
Hu, K. et al. Intramolecular and lateral intermolecular hole transfer at the sensitized TiO2 interface. J. Am. Chem. Soc. 136, 1034–1046 (2014).
Kuciauskas, D., Freund, M. S., Gray, H. B., Winkler, J. R. & Lewis, N. S. Electron transfer dynamics in nanocrystalline titanium dioxide solar cells sensitized with ruthenium or osmium polypyridyl complexes. J. Phys. Chem. B 105, 392–403 (2001).
Ashford, D. L. et al. Photoinduced electron transfer in a chromophore–catalyst assembly anchored to TiO2 . J. Am. Chem. Soc. 134, 19189–19198 (2012).
Barzykin, A. V. & Tachiya, M. Mechanism of molecular control of recombination dynamics in dye-sensitized nanocrystalline semiconductor films. J. Phys. Chem. B 108, 8385–8389 (2004).
Hanson, K. et al. Structure–property relationships in phosphonate-derivatized, RuII polypyridyl dyes on metal oxide surfaces in an aqueous environment. J. Phys. Chem. C 116, 14837–14847 (2012).
Nelson, J. Continuous-time random-walk model of electron transport in nanocrystalline TiO2 electrodes. Phys. Rev. B 59, 15374–15380 (1999).
Huang, Z. J. et al. Dye-controlled interfacial electron transfer for high-current indium tin oxide photocathodes. Angew. Chem. Int. Ed. 54, 6857–6861 (2015).
Hu, K., Robson, K. C. D., Johansson, P. G., Berlinguette, C. P. & Meyer, G. J. Intramolecular hole transfer at sensitized TiO2 interfaces. J. Am. Chem. Soc. 134, 8352–8355 (2012).
Ardo, S., Sun, Y., Staniszewski, A., Castellano, F. N. & Meyer, G. J. Stark effects after excited-state interfacial electron transfer at sensitized TiO2 nanocrystallites. J. Am. Chem. Soc. 132, 6696–6709 (2010).
Brunschwig, B. S., Creutz, C. & Sutin, N. Optical transitions of symmetrical mixed-valence systems in the Class II–III transition regime. Chem. Soc. Rev. 31, 168–184 (2002).
Chen, P. Y. & Meyer, T. J. Medium effects on charge transfer in metal complexes. Chem. Rev. 98, 1439–1477 (1998).
Williams, G. & Watts, D. C. Non-symmetrical dielectric relaxation behaviour arising from a simple empirical decay function. Trans. Faraday Soc. 66, 80–85 (1970).
Lindsey, C. P. & Patterson, G. D. Detailed comparison of the Williams–Watts and Cole–Davidson functions. J. Chem. Phys. 73, 3348–3357 (1980).
Knutson, J. R., Walbridge, D. G. & Brand, L. Decay-associated fluorescence spectra and the heterogeneous emission of alcohol-dehydrogenase. Biochemistry 21, 4671–4679 (1982).
Robson, K. C. D., Koivisto, B. D., Gordon, T. J., Baumgartner, T. & Berlinguette, C. P. Triphenylamine-modified ruthenium(II) terpyridine complexes: enhancement of light absorption by conjugated bridging motifs. Inorg. Chem. 49, 5335–5337 (2010).
Chen, C.-Y. et al. Multifunctionalized ruthenium-based supersensitizers for highly efficient dye-sensitized solar cells. Angew. Chem. Int. Ed. 47, 7342–7345 (2008).
Song, H. E. et al. Linker dependence of energy and hole transfer in neutral and oxidized multiporphyrin arrays. J. Phys. Chem. B 113, 16483–16493 (2009).
Chen, P. Y., Curry, M. & Meyer, T. J. Effects of conformational change in the acceptor on intramolecular electron transfer. Inorg. Chem. 28, 2271–2280 (1989).
Hanss, D., Walther, M. E. & Wenger, O. S. Importance of covalence, conformational effects and tunneling-barrier heights for long-range electron transfer: insights from dyads with oligo-p-phenylene, oligo-p-xylene and oligo-p-dimethoxybenzene bridges. Coord. Chem. Rev. 254, 2584–2592 (2010).
Laine, P. P., Bedioui, F., Loiseau, F., Chiorboli, C. & Campagna, S. Conformationally gated photoinduced processes within photosensitizer–acceptor dyads based on osmium(II) complexes with triarylpyridinio-functionalized terpyridyl ligands: insights from experimental study. J. Am. Chem. Soc. 128, 7510–7521 (2006).
Meylemans, H. A., Lei, C. F. & Damrauer, N. H. Ligand structure, conformational dynamics, and excited-state electron delocalization for control of photoinduced electron transfer rates in synthetic donor–bridge–acceptor systems. Inorg. Chem. 47, 4060–4076 (2008).
Sun, D. L., Rosokha, S. V., Lindeman, S. V. & Kochi, J. K. Intervalence (charge-resonance) transitions in organic mixed-valence systems. Through-space versus through-bond electron transfer between bridged aromatic (redox) centers. J. Am. Chem. Soc. 125, 15950–15963 (2003).
Shen, J.-J. & Zhong, Y.-W. Long-range ruthenium–amine electronic communication through the para-oligophenylene wire. Sci. Rep. 5, 13835 (2015).
Argazzi, R., Bignozzi, C. A., Heimer, T. A., Castellano, F. N. & Meyer, G. J. Enhanced spectral sensitivity from ruthenium(II) polypyridyl based photovoltaic devices. Inorg. Chem. 33, 5741–5749 (1994).
Pavlishchuk, V. V. & Addison, A. W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorg. Chim. Acta 298, 97–102 (2000).
Johansson, P. G. et al. Long-wavelength sensitization of TiO2 by ruthenium diimine compounds with low-lying π* orbitals. Langmuir 27, 14522–14531 (2011).
Frisch, M. J. et al. Gaussian 09 (Gaussian, Inc., 2009).
Robson, K. C. D. et al. Systematic modulation of a bichromic cyclometalated ruthenium(II) scaffold bearing a redox-active triphenylamine constituent. Inorg. Chem. 50, 6019–6028 (2011).
Acknowledgements
The University of North Carolina (UNC) authors gratefully acknowledge support by a grant from the Division of Chemical Sciences, Office of Basic Energy Sciences, Office of Energy Research, US Department of Energy (DE-SC0013461). The University of British Columbia authors are grateful to the Canadian Natural Science and Engineering Research Council, Canadian Foundation for Innovation, Canadian Institute for Advanced Research and Canada Research Chairs for support. The authors thank M. Gish and the Papanikolas group at UNC for the ultrafast measurements.
Author information
Authors and Affiliations
Contributions
G.J.M., C.P.B. and K.H. proposed the ideas, A.D.B. and P.A.S. synthesized the compounds, K.H. and R.N.S. performed the electrochemical and photophysical experiments and K.H., R.N.S. and E.J.P. analysed the data. E.J.P. performed the Mulliken–Hush analysis, F.G.L.P. constructed Fig. 1 and G.J.M. wrote the manuscript with input from all the authors. G.J.M. and C.P.B. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Hu, K., Blair, A., Piechota, E. et al. Kinetic pathway for interfacial electron transfer from a semiconductor to a molecule. Nature Chem 8, 853–859 (2016). https://doi.org/10.1038/nchem.2549
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2549
This article is cited by
-
Photogenerated hole traps in metal-organic-framework photocatalysts for visible-light-driven hydrogen evolution
Communications Chemistry (2022)
-
Design, fabrication, and characterization of thermal and optical properties of nano-composite self-cleaning smart window
Optical and Quantum Electronics (2021)
-
Constructing soft-conjugated materials from small molecules to polymers: a theoretical study
Theoretical Chemistry Accounts (2018)
-
Photoelectrochemical oxidation of organic substrates in organic media
Nature Communications (2017)