Abstract
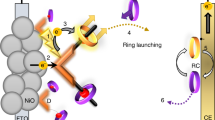
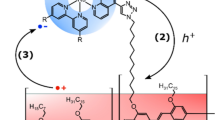
The achievement of long-lived photoinduced redox separation lifetimes has long been a central goal of molecular-based solar energy conversion strategies. The longer the redox-separation lifetime, the more time available for useful work to be extracted from the absorbed photon energy. Here we describe a novel strategy for dye-sensitized solar energy applications in which redox-separated lifetimes on the order of milliseconds to seconds can be achieved based on a simple toolkit of molecular components. Specifically, molecular chromophores (C), electron acceptors (A) and electron donors (D) were self-assembled on the surfaces of mesoporous, transparent conducting indium tin oxide nanoparticle (nanoITO) electrodes to prepare both photoanode (nanoITO|–A–C–D) and photocathode (nanoITO|–D–C–A) assemblies. Nanosecond transient-absorption and steady-state photolysis measurements show that the electrodes function microscopically as molecular analogues of semiconductor p/n junctions. These results point to a new chemical strategy for dye-sensitized solar energy conversion based on molecular excited states and electron acceptors/donors on the surfaces of transparent conducting oxide nanoparticle electrodes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
24 August 2016
In the version of this Article originally published, the affiliation details for Kyung-Ryang Wee were not correct, these have been updated in the online versions of this paper.
References
Shockley, W. & Queisser, H. J. Detailed balance limit of efficiency of p–n junction solar cells. J. Appl. Phys. 32, 510–519 (1961).
Meyer, T. J. Chemical approaches to artificial photosynthesis. Acc. Chem. Res. 22, 163–170 (1989).
Alstrum-Acevedo, J. H., Brennaman, M. K. & Meyer, T. J. Chemical approaches to artificial photosynthesis. 2. Inorg. Chem. 44, 6802–6827 (2005).
Chakraborty, S., Wadas, T. J., Hester, H., Schmehl, R. & Eisenberg, R. Platinum chromophore-based systems for photoinduced charge separation: a molecular design approach for artificial photosynthesis. Inorg. Chem. 44, 6865–6878 (2005).
Kaschak, D. M., Johnson, S. A., Waraksa, C. C., Pogue, J. & Mallouk, T. E. Artificial photosynthesis in lamellar assemblies of metal poly(pyridyl) complexes and metalloporphyrins. Coord. Chem. Rev. 186, 403–416 (1999).
Wasielewski, M. R. Photoinduced electron transfer in supramolecular systems for artificial photosynthesis. Chem. Rev. 92, 435–461 (1992).
Gust, D. et al. Efficient multistep photoinitiated electron transfer in a molecular pentad. Science 248, 199–201 (1990).
Gust, D. & Moore, T. A. Mimicking photosynthesis. Science 244, 35–41 (1989).
Moore, T. A. et al. Photodriven charge separation in a carotenoporphyrin–quinone triad. Nature 307, 630–632 (1984).
Yonemoto, E. H., Riley, R. L., Atherton, S. J., Schmehl, R. H. & Mallouk, T. E. Photoinduced electron transfer in covalently linked ruthenium tris(bipyridyl)–viologen molecules: observation of back electron transfer in the Marcus inverted region. J. Am. Chem. Soc. 114, 8081–8087 (1992).
Sherman, B. D. et al. Evolution of reaction center mimics to systems capable of generating solar fuel. Photosynth. Res. 120, 59–70 (2014).
Massari, A. M. et al. Ultrathin micropatterned porphyrin films assembled via zirconium phosphonate chemistry. Polyhedron 22, 3065–3072 (2003).
Imahori, H. et al. Light-harvesting and photocurrent generation by gold electrodes modified with mixed self-assembled monolayers of boron−dipyrrin and ferrocene−porphyrin−fullerene triad. J. Am. Chem. Soc. 123, 100–110 (2001).
Vermeulen, L. A., Snover, J. L., Sapochak, L. S. & Thompson, M. E. Efficient photoinduced charge separation in layered zirconium viologen phosphonate compounds. J. Am. Chem. Soc. 115, 11767–11774 (1993).
Farnum, B. H., Nakada, A., Ishitani, O. & Meyer, T. J. Bias-dependent oxidative or reductive quenching of a molecular excited-state assembly bound to a transparent conductive oxide. J. Phys. Chem. C 119, 25180–25187 (2015).
Farnum, B. H., Morseth, Z. A., Brennaman, M. K., Papanikolas, J. M. & Meyer, T. J. Driving force dependent, photoinduced electron transfer at degenerately doped, optically transparent semiconductor nanoparticle interfaces. J. Am. Chem. Soc. 136, 15869–15872 (2014).
Farnum, B. H., Morseth, Z. A., Brennaman, M. K., Papanikolas, J. M. & Meyer, T. J. Application of degenerately doped metal oxides in the study of photoinduced interfacial electron transfer. J. Phys. Chem. B 119, 7698–7711 (2015).
Farnum, B. H. et al. Photoinduced interfacial electron transfer within a mesoporous transparent conducting oxide film. J. Am. Chem. Soc. 136, 2208–2211 (2014).
Hoertz, P. G., Chen, Z., Kent, C. A. & Meyer, T. J. Application of high surface area tin-doped indium oxide nanoparticle films as transparent conducting electrodes. Inorg. Chem. 49, 8179–8181 (2010).
Chen, Z. et al. Nonaqueous catalytic water oxidation. J. Am. Chem. Soc. 132, 17670–17673 (2010).
Fang, M., Kaschak, D. M., Sutorik, A. C. & Mallouk, T. E. A ‘mix and match’ ionic−covalent strategy for self-assembly of inorganic multilayer films. J. Am. Chem. Soc. 119, 12184–12191 (1997).
Kaschak, D. M. et al. Photoinduced energy and electron transfer reactions in lamellar polyanion/polycation thin films: toward an inorganic ‘leaf’. J. Am. Chem. Soc. 121, 3435–3445 (1999).
Ishida, T. et al. Self-assembled monolayer and multilayer formation using redox-active Ru complex with phosphonic acids on silicon oxide surface. Appl. Surf. Sci. 255, 8824–8830 (2009).
Akatsuka, K. et al. Photoelectrochemical properties of alternating multilayer films composed of titania nanosheets and Zn porphyrin. Langmuir 23, 6730–6736 (2007).
Katz, H. E. Multilayer deposition of novel organophosphonates with zirconium(IV). Chem. Mater. 6, 2227–2232 (1994).
Ungashe, S. B., Wilson, W. L., Katz, H. E., Scheller, G. R. & Putvinski, T. M. Synthesis, self-assembly, and photophysical dynamics of stacked layers of porphyrin and viologen phosphonates. J. Am. Chem. Soc. 114, 8717–8719 (1992).
Nayak, A. et al. Synthesis and photophysical characterization of porphyrin and porphyrin–Ru(II) polypyridyl chromophore–catalyst assemblies on mesoporous metal oxides. Chem. Sci. 5, 3115–3119 (2014).
Hanson, K. et al. Self-assembled bilayer films of ruthenium(II)/polypyridyl complexes through layer-by-layer deposition on nanostructured metal oxides. Angew. Chem. Int. Ed. 51, 12782–12785 (2012).
Ardo, S. & Meyer, G. J. Photodriven heterogeneous charge transfer with transition-metal compounds anchored to TiO2 semiconductor surfaces. Chem. Soc. Rev. 38, 115–164 (2009).
Hagfeldt, A., Boschloo, G., Sun, L., Kloo, L. & Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 110, 6595–6663 (2010).
Knauf, R. R., Kalanyan, B., Parsons, G. N. & Dempsey, J. L. Charge recombination dynamics in sensitized SnO2/TiO2 core/shell photoanodes. J. Phys. Chem. C 119, 28353–28360 (2015).
Nattestad, A. et al. Highly efficient photocathodes for dye-sensitized tandem solar cells. Nature Mater. 9, 31–35 (2010).
Weidelener, M. et al. Synthesis and characterization of perylene–bithiophene–triphenylamine triads: studies on the effect of alkyl-substitution in p-type NiO based photocathodes. J. Mater. Chem. 22, 7366–7379 (2012).
Gibson, E. A. et al. A p-type NiO-based dye-sensitized solar cell with an open-circuit voltage of 0.35 V. Angew. Chem. Int. Ed. 48, 4402–4405 (2009).
Warnan, J. et al. Multichromophoric sensitizers based on squaraine for NiO based dye-sensitized solar cells. J. Phys. Chem. C 118, 103–113 (2014).
Zhang, L. et al. Long-lived charge separated state in NiO-based p-type dye-sensitized solar cells with simple cyclometalated iridium complexes. J. Phys. Chem. Lett. 5, 2254–2258 (2014).
Felderhoff, M., Heinen, S., Molisho, N., Webersinn, S. & Walder, L. Molecular suppression of the pimerization of viologens attached to nanocrystalline titanium dioxide thin-film electrodes. Helv. Chim. Acta 83, 181–192 (2000).
Hanson, K. et al. Structure–property relationships in phosphonate-derivatized, Ru(II) polypyridyl dyes on metal oxide surfaces in an aqueous environment. J. Phys. Chem. C 116, 14837–14847 (2012).
Watanabe, T. & Honda, K. Measurement of the extinction coefficient of the methyl viologen cation radical and the efficiency of its formation by semiconductor photocatalysis. J. Phys. Chem. 86, 2617–2619 (1982).
Lapides, A. M. et al. Synthesis, characterization, and water oxidation by a molecular chromophore–catalyst assembly prepared by atomic layer deposition. The ‘mummy’ strategy. Chem. Sci. 6, 6398–6406 (2015).
Danielson, E., Elliott, C. M., Merkert, J. W. & Meyer, T. J. Photochemically induced charge separation at the molecular level. A chromophore quencher complex containing both an electron transfer donor and an acceptor. J. Am. Chem. Soc. 109, 2519–2520 (1987).
Treadway, J. A., Chen, P., Rutherford, T. J., Keene, F. R. & Meyer, T. J. Mapping electron transfer pathways in a chromophore–quencher triad. J. Phys. Chem. A 101, 6824–6826 (1997).
Nelson, J. Continuous-time random-walk model of electron transport in nanocrystalline TiO2 electrodes. Phys. Rev. B 59, 374–380 (1999).
Lindsey, C. P. & Patterson, G. D. Detailed comparison of the Williams–Watts and Cole–Davidson functions. J. Chem. Phys. 73, 3348–3357 (1980).
Williams, G. & Watts, D. C. Non-symmetrical dielectric relaxation behaviour arising from a simple empirical decay function. Trans. Faraday Soc. 66, 80–85 (1969).
Ferreira, K. N., Iverson, T. M., Maghlaoui, K., Barber, J. & Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004).
Huang, Z. et al. Dye-controlled interfacial electron transfer for high-current indium tin oxide photocathodes. Angew. Chem. Int. Ed. 54, 6857–6861 (2015).
Acknowledgements
This material is based on work supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Award No. DE-FG02-06ER15788.
Author information
Authors and Affiliations
Contributions
B.H.F. and T.J.M. conceived and designed the experiments. K.R.W. synthesized the molecular species. B.H.F. assembled the electrodes and performed the transient-absorption and photolysis experiments. B.H.F. and T.J.M. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Farnum, B., Wee, KR. & Meyer, T. Self-assembled molecular p/n junctions for applications in dye-sensitized solar energy conversion. Nature Chem 8, 845–852 (2016). https://doi.org/10.1038/nchem.2536
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2536
This article is cited by
-
Binary molecular-semiconductor p–n junctions for photoelectrocatalytic CO2 reduction
Nature Energy (2019)
-
Direct observation of sequential oxidations of a titania-bound molecular proxy catalyst generated through illumination of molecular sensitizers
Nature Chemistry (2018)
-
Bay-Region Functionalisation of Ar-BIAN Ligands and Their Use Within Highly Absorptive Cationic Iridium(III) Dyes
Scientific Reports (2017)
-
A low-spin Fe(iii) complex with 100-ps ligand-to-metal charge transfer photoluminescence
Nature (2017)
-
Erratum: Corrigendum: Self-assembled molecular p/n junctions for applications in dye-sensitized solar energy conversion
Nature Chemistry (2016)