Abstract
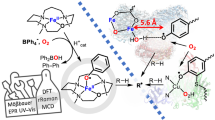
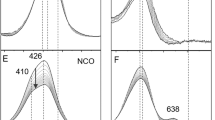
Nitric oxide (NO) participates in numerous biological processes, such as signalling in the respiratory system and vasodilation in the cardiovascular system. Many metal-mediated processes involve direct reaction of NO to form a metal–nitrosyl (M–NO), as occurs at the Fe2+ centres of soluble guanylate cyclase or cytochrome c oxidase. However, some copper electron-transfer proteins that bear a type 1 Cu site (His2Cu–Cys) reversibly bind NO by an unknown motif. Here, we use model complexes of type 1 Cu sites based on tris(pyrazolyl)borate copper thiolates [CuII]-SR to unravel the factors involved in NO reactivity. Addition of NO provides the fully characterized S-nitrosothiol adduct [CuI](κ1-N(O)SR), which reversibly loses NO on purging with an inert gas. Computational analysis outlines a low-barrier pathway for the capture and release of NO. These findings suggest a new motif for reversible binding of NO at bioinorganic metal centres that can interconvert NO and RSNO molecular signals at copper sites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Ignarro, L. J. Nitric Oxide, Biology and Pathobiology 2nd edn (Academic, 2010).
Cary, S. P. L., Winger, J. A., Derbyshire, E. R. & Marletta, M. A. Nitric oxide signaling: no longer simply on or off. Trends Biochem. Sci. 31, 231–239 (2006).
Francis, S. H., Busch, J. L. & Corbin, J. D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 62, 525–563 (2010).
Hematian, S. et al. Nitrogen oxide atom-transfer redox chemistry; mechanism of NO(g) to nitrite conversion utilizing μ-oxo heme-FeIII–O–CuII(L) constructs. J. Am. Chem. Soc. 137, 6602–6615 (2015).
Mason, M. G., Nicholls, P., Wilson, M. T. & Cooper, C. E. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc. Natl Acad. Sci. USA 103, 708–713 (2006).
Brown, G. C. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 369, 136–139 (1995).
Ohta, K. et al. X-ray structure of the NO-bound CuB in bovine cytochrome c oxidase. Acta Crystallogr. F 66, 251–253 (2010).
Tocheva, E. I., Rosell, F. I., Mauk, A. G. & Murphy, M. E. P. Side-on copper nitrosyl coordination by nitrite reductase. Science 304, 867–870 (2004).
Merkle, A. C. & Lehnert, N. Binding and activation of nitrite and nitric oxide by copper nitrite reductase and corresponding model complexes. Dalton Trans. 41, 3355–3368 (2012).
Usov, O. M., Sun, Y., Grigoryants, V. M., Shapleigh, J. P. & Scholes, C. P. EPR–ENDOR of the Cu(I)NO complex of nitrite reductase. J. Am. Chem. Soc. 128, 13102–13111 (2006).
Fujisawa, K. et al. Structural and spectroscopic characterization of mononuclear copper(I) nitrosyl complexes: end-on versus side-on coordination of NO to copper(I). J. Am. Chem. Soc. 130, 1205–1213 (2008).
Wever, R., Van Leeuwen, F. X. R. & Van Gelder, B. F. The reaction of nitric oxide with ceruloplasmin. Biochim. Biophys. Acta 302, 236–239 (1973).
Gorren, A. C. F., De Boer, E. & Wever, R. The reaction of nitric oxide with copper proteins and the photodissociation of copper–nitric oxide complexes. Biochim. Biophys. Acta 916, 38–47 (1987).
Van Leeuwen, F. X. R., Wever, R., Van Gelder, B. F., Avigliano, L. & Mondovi, B. The interaction of nitric oxide with ascorbate oxidase. Biochim. Biophys. Acta 403, 285–291 (1975).
Ehrenstein, D., Filiaci, M., Scharf, B., Engelhard, M. & Nienhaus, G. U. Ligand binding and protein dynamics in cupredoxins. Biochemistry 34, 12170–12177 (1995).
Solomon, E. I. et al. Copper active sites in biology. Chem. Rev. 114, 3659–3853 (2014).
Liu, J. et al. Metalloproteins containing cytochrome, iron–sulfur, or copper redox centers. Chem. Rev. 114, 4366–4469 (2014).
Solomon, E. I., Szilagyi, R. K., DeBeer George, S. & Basumallick, L. Electronic structures of metal sites in proteins and models: contributions to function in blue copper proteins. Chem. Rev. 104, 419–458 (2004).
Inoue, K. et al. Nitrosothiol formation catalyzed by ceruloplasmin. J. Biol. Chem. 274, 27069–27075 (1999).
Shiva, S. et al. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nature Chem. Biol. 2, 486–493 (2006).
Rassaf, T. et al. Plasma nitrosothiols contribute to the systemic vasodilator effects of intravenously applied NO. Experimental and clinical study on the fate of NO in human blood. Circ. Res. 91, 470–477 (2002).
Stamler, J. S. & Toone, E. J. The decomposition of thionitrites. Curr. Opin. Chem. Biol. 6, 779–785 (2002).
Zhang, S., Çelebi-Ölçüm, N., Melzer, M. M., Houk, K. N. & Warren, T. H. Copper(I) nitrosyls from reaction of copper(II) thiolates with S-nitrosothiols: mechanism of NO release from RSNOs at Cu. J. Am. Chem. Soc. 135, 16746–16749 (2013).
Hess, D. T., Matsumoto, A., Kim, S.-O., Marshall, H. E. & Stamler, J. S. Protein S-nitrosylation: purview and parameters. Nature Rev. Mol. Cell Biol. 6, 150–166 (2005).
Foster, M. W., Hess, D. T. & Stamler, J. S. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol. Med. 15, 391–404 (2009).
Kim, S. F., Huri, D. A. & Snyder, S. H. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310, 1966–1970 (2005).
Johnson, M. A., Macdonald, T. L., Mannick, J. B., Conaway, M. R. & Gaston, B. Accelerated S-nitrosothiol breakdown by amyotrophic lateral sclerosis mutant copper, zinc-superoxide dismutase. J. Biol. Chem. 276, 39872–39878 (2001).
Schonhoff, C. M. et al. S-nitrosothiol depletion in amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 103, 2404–2409 (2006).
Romeo, A. A., Filosa, A., Capobianco, J. A. & English, A. M. Metal chelators inhibit S-nitrosation of Cysβ93 in oxyhemoglobin. J. Am. Chem. Soc. 123, 1782–1783 (2001).
Pawloski, J. R., Hess, D. T. & Stamler, J. S. Export by red blood cells of nitric oxide bioactivity. Nature 409, 622–626 (2001).
McMahon, T. J. et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc. Natl Acad. Sci. USA 102, 14801–14806 (2005).
Ford, P. C. & Lorkovic, I. M. Mechanistic aspects of the reactions of nitric oxide with transition-metal complexes. Chem. Rev. 102, 993–1018 (2002).
McCleverty, J. A. Chemistry of nitric oxide relevant to biology. Chem. Rev. 104, 403–418 (2004).
Ford, P. C., Fernandez, B. O. & Lim, M. D. Mechanisms of reductive nitrosylation in iron and copper models relevant to biological systems. Chem. Rev. 105, 2439–2455 (2005).
Kitajima, N., Fujisawa, K., Tanaka, M. & Morooka, Y. X-ray structure of thiolatocopper(II) complexes bearing close spectroscopic similarities to blue copper proteins. J. Am. Chem. Soc. 114, 9232–9233 (1992).
Qiu, D., Kilpatrick, L., Kitajima, N. & Spiro, T. G. Modeling blue copper protein resonance Raman spectra with thiolate–CuII complexes of a sterically hindered tris(pyrazolyl)borate. J. Am. Chem. Soc. 116, 2585–2590 (1994).
Randall, D. W. et al. Spectroscopic and electronic structural studies of blue copper model complexes. Part 1. Perturbation of the thiolate–Cu bond. J. Am. Chem. Soc. 122, 11620–11631 (2000).
Arulsamy, N. et al. Interrelationships between conformational dynamics and the redox chemistry of S-nitrosothiols. J. Am. Chem. Soc. 121, 7115–7123 (1999).
Fujisawa, K. et al. Structural and electronic differences of copper(I) complexes with tris(pyrazolyl)methane and hydrotris(pyrazolyl)borate ligands. Inorg. Chem. 45, 1698–1713 (2006).
Rheingold, A. L., White, C. B. & Trofimenko, S. Hydrotris(3-mesitylpyrazol-1-yl)borate and hydrobis(3-mesitylpyrazol-1-yl)(5-mesitylpyrazol-1-yl)borate: symmetric and asymmetric ligands with rotationally restricted aryl substituents. Inorg. Chem. 32, 3471–3477 (1993).
Schneider, J. L., Carrier, S. M., Ruggiero, C. E., Young, V. G. & Tolman, W. B. Influences of ligand environment on the spectroscopic properties and disproportionation reactivity of copper–nitrosyl complexes. J. Am. Chem. Soc. 120, 11408–11418 (1998).
Perissinotti, L. L., Estrin, D. A., Leitus, G. & Doctorovich, F. A surprisingly stable S-nitrosothiol complex. J. Am. Chem. Soc. 128, 2512–2513 (2006).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Frisch, M. J. Gaussian 09, Revision D.01 (Gaussian, 2009).
Baciu, C., Cho, K.-B. & Gauld, J. W. Influence of Cu+ on the RS–NO bond dissociation energy of S-nitrosothiols. J. Phys. Chem. B 109, 1334–1336 (2005).
Wright, A. M., Wu, G. & Hayton, T. W. Structural characterization of a copper nitrosyl complex with a {CuNO}10 configuration. J. Am. Chem. Soc. 132, 14336–14337 (2010).
Williams, D. L. H. The chemistry of S-nitrosothiols. Acc. Chem. Res. 32, 869–876 (1999).
Schwane, J. D. & Ashby, M. T. FTIR investigation of the intermediates formed in the reaction of nitroprusside and thiolates. J. Am. Chem. Soc. 124, 6822–6823 (2002).
Perissinotti, L. L., Turjanski, A. G., Estrin, D. A. & Doctorovich, F. Transnitrosation of nitrosothiols: characterization of an elusive intermediate. J. Am. Chem. Soc. 127, 486–487 (2005).
Melzer, M. M., Li, E. & Warren, T. H. Reversible RS–NO bond cleavage and formation at copper(I) thiolates. Chem. Commun. 5847–5849 (2009).
Acknowledgements
This material is based on work supported by the US National Science Foundation (award no. CHE-1459090, to T.H.W.). The authors thank the Purdue University Department of Chemistry and the Vorisek Endowment at Georgetown University for additional financial support (to T.H.W.), as well as the TUBITAK ULAKBIM High Performance and Grid Computing Center (TRUBA, Turkey) for computer time (N.Ç.-O.).
Author information
Authors and Affiliations
Contributions
S.Z., N.Ç.-Ö. and T.H.W. conceived and designed the research. S.Z., M.M.M., S.N.S. and N.Ç.-Ö. collected data and performed calculations. S.Z., M.M.M., S.N.S., N.Ç.-Ö. and T.H.W. analysed the data. S.Z., N.Ç.-Ö. and T.H.W. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5913 kb)
Supplementary information
Crystallographic data for compound MesTpCu-SCPh3 (1b) (CIF 1080 kb)
Supplementary information
Crystallographic data for compound MesTpCu(κ1-N(O)SCPh3) (2b) (CIF 1541 kb)
Supplementary information
Crystallographic data for compound iPr2TpCuI(CNAr2,6-Me2) (3a) (CIF 1461 kb)
Rights and permissions
About this article
Cite this article
Zhang, S., Melzer, M., Sen, S. et al. A motif for reversible nitric oxide interactions in metalloenzymes. Nature Chem 8, 663–669 (2016). https://doi.org/10.1038/nchem.2502
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2502
This article is cited by
-
Effect of exogenous nitric oxide on sulfur and nitrate assimilation pathway enzymes in maize (Zea mays L.) under drought stress
Acta Physiologiae Plantarum (2018)
-
A switch for blue copper proteins?
Nature Chemistry (2016)