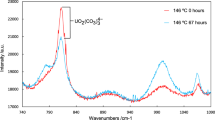
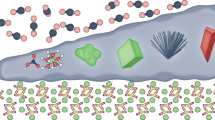
Abstract
The reactivity, speciation and solvation structure of CO2 in carbonate melts are relevant for both the fate of carbon in deep geological formations and for its electroreduction to CO (to be used as fuel) when solvated in a molten carbonate electrolyte. In particular, the high solubility of CO2 in carbonate melts has been tentatively attributed to the formation of the pyrocarbonate anion, C2O52–. Here we study, by first-principles molecular dynamics simulations, the behaviour of CO2 in molten calcium carbonate. We find that pyrocarbonate forms spontaneously and the identity of the CO2 molecule is quickly lost through O2– exchange. The transport of CO2 in this molten carbonate thus occurs in a fashion similar to the Grotthuss mechanism in water, and is three times faster than molecular diffusion. This shows that Grotthuss-like transport is more general than previously thought.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gaillard, F., Malki, M., Iacono-Marziano, G., Pichavant, M. & Scaillet, B. Carbonatite melts and electrical conductivity in the asthenosphere. Science 322, 1363–1365 (2008).
Jones, A. P., Genge, M. & Carmody, L. Carbonate melts and carbonatites. Rev. Mineral. Geochem. 75, 289–322 (2013).
Kaminsky, F., Wirth, R., Schreiber, A. & Thomas, R. Nyerereite and nahcolite inclusions in diamond: evidence for lower-mantle carbonatitic melts. Mineral. Mag. 73, 797–816 (2009).
Stoppa, F., Jones, A. P. & Sharygin, V. Nyerereite from carbonatite rocks at Vulture volcano: implications for mantle metasomatism and petrogenesis of alkali carbonate melts. Cent. Euro. J. Geosci. 1, 131–151 (2009).
Kaminsky, F. Mineralogy of the lower mantle: a review of ‘super-deep’ mineral inclusions in diamond. Earth Sci. Rev. 110, 127–147 (2012).
Dasgupta, R. & Hirschmann, M. M. The deep carbon cycle and melting in Earth's interior. Earth Planet. Sci. Lett. 298, 1–13 (2010).
Dasgupta, R. & Hirschmann, M. M. Melting in Earth's deep upper mantle caused by carbon dioxide. Nature 440, 659–662 (2006).
Chery, D., Lair, V. & Cassir, M. CO2 electrochemical reduction into CO or C in molten carbonates: a thermodynamic point of view. Electrochim. Acta 160, 74–81 (2015).
Chery, D., Albin, V., Lair, V. & Cassir, M. Thermodynamic and experimental approach of electrochemical reduction of CO2 in molten carbonates. Int. J. Hydrogen Energy 39, 12330–12339 (2014).
Kanai, Y., Fukunaga, K., Terasaka, K. & Fujioka, S. Mass transfer in molten salt and suspended molten salt in bubble column. Chem. Eng. Sci. 100, 153–159 (2013).
Claes, P., Moyaux, D. & Peeters, D. Solubility and solvation of carbon dioxide in the molten Li2CO3/Na2CO3/K2CO3 (43.5:31.5:25.0 mol-%) eutectic mixture at 973K. I. Experimental part. Eur. J. Inorg. Chem. 1999, 583–588 (1999).
Peeters, D., Moyaux, D. & Claes, P. Solubility and solvation of carbon dioxide in the molten Li2CO3/Na2CO3/K2CO3 (43.5:31.5:25.0 mol-%) eutectic mixture at 973K. II. Theoretical part. Eur. J. Inorg. Chem. 1999, 589–592 (1999).
Burna, P. J., Grein, F. & Passmore, J. Density functional theory (DFT) calculations on the structures and stabilities of [CnO2n+1]2– and [CnO2n+1]X2 polycarbonates containing chainlike (CO2)n units (n=2–6; X=H or Li). Can. J. Chem. 89, 671–687 (2011).
Frapper, G. & Saillard, J.-Y. Search for new allotropic forms of carbon dioxide and carbon disulfide: a density functional study of CX2-based oligomers (X= O, S). J. Am. Chem. Soc. 122, 5367–5370 (2000).
Zhang, L. et al. First spectroscopic identification of pyrocarbonate for high CO2 flux membranes containing highly interconnected three dimensional ionic channels. Phys. Chem. Chem. Phys. 15, 13147–13152 (2013).
Zeller, K.-P., Schuler, P. & Haiss, P. The hidden equilibrium in aqueous sodium carbonate solutions—evidence for the formation of the dicarbonate anion. Eur. J. Inorg. Chem. 168–172 (2005).
Vuilleumier, R., Seitsonen, A., Sator, N. & Guillot, B. Structure, equation of state and transport properties of molten calcium carbonate (CaCO3) by atomistic simulations. Geochim. Cosmochim. Acta. 141, 547–566 (2014).
Vuilleumier, R., Seitsonen, A. P., Sator, N. & Guillot, B. Carbon dioxide in silicate melts at upper mantle conditions: insights from atomistic simulations. Chem. Geol. 418, 77–88 (2015).
Saitta, A. M., Saija, F. & Giaquinta, P. V. Ab initio molecular dynamics study of dissociation of water under an electric field. Phys. Rev. Lett. 108, 207801 (2012).
Kelemen, Z. et al. An abnormal N-heterocyclic carbene–carbon dioxide adduct from imidazolium acetate ionic liquids: the importance of basicity. Chem. Eur. J. 20, 13002–13008 (2014).
Chery, D., Lair, V. & Cassir, M. Overview on CO2 valorization: challenge of molten carbonates. Front. Energy Res. 3, 43 (2015).
Nosé, S. A molecular-dynamics method for simulations in the canonical ensemble. Mol. Phys. 52, 255–268 (1984).
Nosé, S. A unified formulation of the constant temperature molecular-dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Suito, K. et al. Phase relations of CaCO3 at high pressure and high temperature. Am. Mineral. 86, 997–1002 (2001).
Spivak, A. V., Litvin, Y. A., Ovsyannikov, S. V., Dubrovinskaia, N. A. & Dubrovinsky, L. S. Stability and breakdown of Ca13CO3 melt associated with formation of 13C-diamond in static high pressure experiments up to 43 GPa and 3900 K. Solid State Chem. 191, 102–106 (2012).
Hutter, J., Iannuzzi, M., Schiffmann, F. & VandeVondele, J. CP2K: atomistic simulations of condensed matter systems. WIREs Comput. Mol. Sci. 4, 15–25 (2014).
VandeVondele, J. et al. QUICKSTEP: fast and accurate density functional calculations using a mixed Gaussian and plane waves. Comp. Phys. Commun. 167, 103–128 (2005).
Lippert, G., Hutter, J. & Parrinello, M. A hybrid Gaussian and plane wave density functional scheme. Mol. Phys. 92, 477–487 (1997).
Goedecker, S., Teter, M. & Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 54, 1703–1710 (1996).
Hartwigsen, C., Goedecker, S. & Hutter, J. Relativistic separable dual-space Gaussian pseudopotentials from H to Rn. Phys. Rev. B 58, 3641–3662 (1998).
Krack, M. Pseudopotentials for H to Kr optimized for gradient-corrected exchange-correlation functionals. Theor. Chem. Acc. 114, 145–152 (2005).
VandeVondele, J. et al. The influence of temperature and density functional models in ab initio molecular dynamics simulation of liquid water. J. Chem. Phys. 122, 014515 (2005).
VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Cormen, T. H., Leiserson, C. E., Rivest, R. L. & Stein, C. Introduction to Algorithms (MIT Press, 1990).
Laage, D. & Hynes, J. T. A molecular jump mechanism of water reorientation. Science 311, 832–835 (2006).
Humphrey, W., Dalke, A. & Schulten, K. VMD—visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Acknowledgements
The authors thank M. Cassir, V. Lair, B. Guillot, F. Gaillard, V. Haigis and A. Boutin for discussions. The research reported herein was funded by PSL Research University (project COOCAR, grant ANR-10-IDEX-0001-02) and Agence Nationale de la Recherche (project ELECTROLITH, grant ANR-2010-BLAN-621-03). This work was performed using HPC resources from GENCI (grants 2013-082309, 2014-082309 and 2015-082309) and IDRIS (grant ‘Grand Challenge’ 100577). The authors acknowledge PRACE for awarding access to Resource Curie, based in France at CEA Bruyères-le-Chatel (preparatory access allocation 2010PA2746).
Author information
Authors and Affiliations
Contributions
R.V. performed the FPMD simulations. D.C. analysed the trajectories, prepared the figures and wrote the manuscript. All authors designed the research, discussed the results and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 369 kb)
Rights and permissions
About this article
Cite this article
Corradini, D., Coudert, FX. & Vuilleumier, R. Carbon dioxide transport in molten calcium carbonate occurs through an oxo-Grotthuss mechanism via a pyrocarbonate anion. Nature Chem 8, 454–460 (2016). https://doi.org/10.1038/nchem.2450
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2450
This article is cited by
-
CO3+1 network formation in ultra-high pressure carbonate liquids
Scientific Reports (2019)
-
Molecular insights into the carbon dioxide–carboxylate anion interactions and implications for carbon capture
Theoretical Chemistry Accounts (2019)
-
Liquid metal–organic frameworks
Nature Materials (2017)
-
Catch the carbon dioxide
Nature Chemistry (2016)