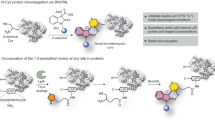
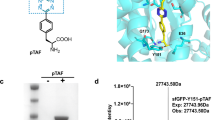
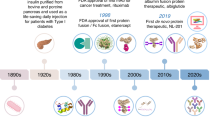
Abstract
Nature has produced intricate machinery to covalently diversify the structure of proteins after their synthesis in the ribosome. In an attempt to mimic nature, chemists have developed a large set of reactions that enable post-expression modification of proteins at pre-determined sites. These reactions are now used to selectively install particular modifications on proteins for many biological and therapeutic applications. For example, they provide an opportunity to install post-translational modifications on proteins to determine their exact biological roles. Labelling of proteins in live cells with fluorescent dyes allows protein uptake and intracellular trafficking to be tracked and also enables physiological parameters to be measured optically. Through the conjugation of potent cytotoxicants to antibodies, novel anti-cancer drugs with improved efficacy and reduced side effects may be obtained. In this Perspective, we highlight the most exciting current and future applications of chemical site-selective protein modification and consider which hurdles still need to be overcome for more widespread use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Walsh, C. T., Garneau-Tsodikova, S. & Gatto, G. J. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew. Chem. Int. Ed. 44, 7342–7372 (2005).
Stephanopoulos, N. & Francis, M. B. Choosing an effective protein bioconjugation strategy. Nature Chem. Biol. 7, 876–884 (2011).
Chalker, J. M., Bernardes, G. J. L., Lin, Y. A. & Davis, B. G. Chemical modification of proteins at cysteine: opportunities in chemistry and biology. Chem. Asian J. 4, 630–640 (2009).
Gaertner, H. F. & Offord, R. E. Site-specific attachment of functionalized poly(ethylene glycol) to the amino terminus of proteins. Bioconjugate Chem. 7, 38–44 (1996).
Dawson, P., Muir, T., Clark-Lewis, I. & Kent, S. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Xie, J. & Schultz, P. G. A chemical toolkit for proteins — an expanded genetic code. Nature Rev. Mol. Cell Biol. 7, 775–782 (2006).
Davis, L. & Chin, J. W. Designer proteins: applications of genetic code expansion in cell biology. Nature Rev. Mol. Cell Biol. 13, 168–182 (2012).
Lang, K. & Chin, J. W. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins. Chem. Rev. 114, 4764–4806 (2014).
Sletten, E. M. & Bertozzi, C. R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 48, 6974–6998 (2009).
Elliott, T. S. et al. Proteome labeling and protein identification in specific tissues and at specific developmental stages in an animal. Nature Biotech. 32, 465–472 (2014).
Schumacher, D. & Hackenberger, C. P. R. More than add-on: chemoselective reactions for the synthesis of functional peptides and proteins. Curr. Opin. Chem. Biol. 22, 62–69 (2014).
Spicer, C. D. & Davis, B. G. Selective chemical protein modification. Nature Commun. 5, 4740 (2014).
Arur, S. & Schedl, T. Generation and purification of highly specific antibodies for detecting post-translationally modified proteins in vivo. Nature Protoc. 9, 375–395 (2014).
Burckstummer, T. et al. An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nature Methods 3, 1013–1019 (2006).
Wang, P. et al. Erythropoietin derived by chemical synthesis. Science 342, 1357–1360 (2013).
Muir, T. W., Sondhi, D. & Cole, P. A. Expressed protein ligation: a general method for protein engineering. Proc. Natl Acad. Sci. USA 95, 6705–6710 (1998).
Fierz, B. & Muir, T. W. Chromatin as an expansive canvas for chemical biology. Nature Chem. Biol. 8, 417–427 (2012).
Casadio, F. et al. H3R42me2a is a histone modification with positive transcriptional effects. Proc. Natl Acad. Sci. USA 110, 14894–14899 (2013).
Kee, J.-M., Oslund, R. C., Perlman, D. H. & Muir, T. W. A pan-specific antibody for direct detection of protein histidine phosphorylation. Nature Chem. Biol. 9, 416–421 (2013).
Chalker, J. M. et al. Methods for converting cysteine to dehydroalanine on peptides and proteins. Chem. Sci. 2, 1666–1676 (2011).
Chooi, K. P. et al. Synthetic phosphorylation of p38α recapitulates protein kinase activity. J. Am. Chem. Soc. 136, 1698–1701 (2014).
Serwa, R. et al. Chemoselective Staudinger-phosphite reaction of azides for the phosphorylation of proteins. Angew. Chem. Int. Ed. 48, 8234–8239 (2009).
Chalker, J. M., Lercher, L., Rose, N. R., Schofield, C. J. & Davis, B. G. Conversion of cysteine into dehydroalanine enables access to synthetic histones bearing diverse post-translational modifications. Angew. Chem. Int. Ed. 51, 1835–1839 (2012).
Simon, M. D. et al. The site-specific installation of methyl-lysine analogs into recombinant histones. Cell 128, 1003–1012 (2007).
Huang, R. et al. Site-specific introduction of an acetyl-lysine mimic into peptides and proteins by cysteine alkylation. J. Am. Chem. Soc. 132, 9986–9987 (2010).
Chatterjee, C., McGinty, R. K., Fierz, B. & Muir, T. W. Disulfide-directed histone ubiquitylation reveals plasticity in hDot1L activation. Nature Chem. Biol. 6, 267–269 (2010).
Li, F. et al. A direct method for site-specific protein acetylation. Angew. Chem. Int. Ed. 50, 9611–9614 (2011).
Virdee, S. et al. Traceless and site-specific ubiquitination of recombinant proteins. J. Am. Chem. Soc. 133, 10708–10711 (2011).
Virdee, S., Ye, Y., Nguyen, D. P., Komander, D. & Chin, J. W. Engineered diubiquitin synthesis reveals Lys29-isopeptide specificity of an OTU deubiquitinase. Nature Chem. Biol. 6, 750–757 (2010).
Madrzak, J. et al. Ubiquitination of the dishevelled DIX domain blocks its head-to-tail polymerization. Nature Commun. 6, 6718 (2015).
Schneider, T. et al. Dissecting ubiquitin signaling with linkage-defined and protease resistant ubiquitin chains. Angew. Chem. Int. Ed. 53, 12925–12929 (2014).
Hemantha, H. P. et al. Nonenzymatic polyubiquitination of expressed proteins. J. Am. Chem. Soc. 136, 2665–2673 (2014).
van Kasteren, S. I. et al. Expanding the diversity of chemical protein modification allows post-translational mimicry. Nature 446, 1105–1109 (2007).
Neumann, H. et al. A method for genetically installing site-specific acetylation in recombinant histones defines the effects of H3 K56 acetylation. Mol. Cell 36, 153–163 (2009).
Perols, A. et al. Influence of DOTA chelator position on biodistribution and targeting properties of 111In-labeled synthetic anti-HER2 affibody molecules. Bioconjugate Chem. 23, 1661–1670 (2012).
Junutula, J. R. et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nature Biotech. 26, 925–932 (2008).
Sachdeva, A., Wang, K., Elliott, T. & Chin, J. W. Concerted, rapid, quantitative, and site-specific dual labeling of proteins. J. Am. Chem. Soc. 136, 7785–7788 (2014).
Tyagi, S. & Lemke, E. A. in Methods in Cell Biology, Vol. 113 (ed. Michael Conn, P.) Ch. 9, 169–187 (Academic Press, 2013).
Wang, K. et al. Optimized orthogonal translation of unnatural amino acids enables spontaneous protein double-labelling and FRET. Nature Chem. 6, 393–403 (2014).
Lang, K. et al. Genetically encoded norbornene directs site-specific cellular protein labelling via a rapid bioorthogonal reaction. Nature Chem. 4, 298–304 (2012).
Zheng, S., Zhang, G., Li, J. & Chen, P. R. Monitoring endocytic trafficking of anthrax lethal factor by precise and quantitative protein labeling. Angew. Chem. Int. Ed. 53, 6449–6453 (2014).
Zeglis, B. M. et al. A pretargeted pet imaging strategy based on bioorthogonal Diels–Alder click chemistry. J. Nucl. Med. 54, 1389–1396 (2013).
Rashidian, M. et al. Use of 18F-2-fluorodeoxyglucose to label antibody fragments for immuno-positron emission tomography of pancreatic cancer. ACS Cent. Sci. 1, 142–147 (2015).
Rossin, R., Läppchen, T., van den Bosch, S. M., Laforest, R. & Robillard, M. S. Diels–Alder reaction for tumor pretargeting: in vivo chemistry can boost tumor radiation dose compared with directly labeled antibody. J. Nucl. Med. 54, 1989–1995 (2013).
Seitchik, J. L. et al. Genetically encoded tetrazine amino acid directs rapid site-specific in vivo bioorthogonal ligation with trans-cyclooctenes. J. Am. Chem. Soc. 134, 2898–2901 (2012).
Yang, M. et al. Converting a solvatochromic fluorophore into a protein-based pH indicator for extreme acidity. Angew. Chem. Int. Ed. 51, 7674–7679 (2012).
Chari, R. V. J., Miller, M. L. & Widdison, W. C. Antibody–drug conjugates: an emerging concept in cancer therapy. Angew. Chem. Int. Ed. 53, 3796–3827 (2014).
Pasut, G. & Veronese, F. M. PEG conjugates in clinical development or use as anticancer agents: an overview. Adv. Drug Deliv. Rev. 61, 1177–1188 (2009).
Alconcel, S. N. S., Baas, A. S. & Maynard, H. D. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym. Chem. 2, 1442–1448 (2011).
Keefe, A. J. & Jiang, S. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nature Chem. 4, 59–63 (2012).
Pelegri-O'Day, E. M., Lin, E.-W. & Maynard, H. D. Therapeutic protein–polymer conjugates: advancing beyond pegylation. J. Am. Chem. Soc. 136, 14323–14332 (2014).
Gilmore, J. M., Scheck, R. A., Esser-Kahn, A. P., Joshi, N. S. & Francis, M. B. N-terminal protein modification through a biomimetic transamination reaction. Angew. Chem. Int. Ed. 45, 5307–5311 (2006).
MacDonald, J. I., Munch, H. K., Moore, T. & Francis, M. B. One-step site-specific modification of native proteins with 2-pyridinecarboxyaldehydes. Nature Chem. Biol. 11, 326–331 (2015).
Cho, H. et al. Optimized clinical performance of growth hormone with an expanded genetic code. Proc. Natl Acad. Sci. USA 108, 9060–9065 (2011).
Chari, R. V. J. et al. Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res. 52, 127–131 (1992).
Beckley, N. S., Lazzareschi, K. P., Chih, H.-W., Sharma, V. K. & Flores, H. L. Investigation into temperature-induced aggregation of an antibody drug conjugate. Bioconjugate Chem. 24, 1674–1683 (2013).
Hamblett, K. J. et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 10, 7063–7070 (2004).
Shen, B.-Q. et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nature Biotech. 30, 184–189 (2012).
Steiner, M. et al. Spacer length shapes drug release and therapeutic efficacy of traceless disulfide-linked ADCs targeting the tumor neovasculature. Chem. Sci. 4, 297–302 (2013).
Cal, P. M. S. D., Bernardes, G. J. L. & Gois, P. M. P. Cysteine-selective reactions for antibody conjugation. Angew. Chem. Int. Ed. 53, 10585–10587 (2014).
Lyon, R. P. et al. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nature Biotech. 32, 1059–1062 (2014).
Tumey, L. N. et al. Mild method for succinimide hydrolysis on ADCs: impact on adc potency, stability, exposure, and efficacy. Bioconjugate Chem. 25, 1871–1880 (2014).
Toda, N., Asano, S. & Barbas, C. F. Rapid, stable, chemoselective labeling of thiols with Julia-Kocieński-like reagents: a serum-stable alternative to maleimide-based protein conjugation. Angew. Chem. Int. Ed. 52, 12592–12596 (2013).
Bernardes, G. J. L., Chalker, J. M., Errey, J. C. & Davis, B. G. Facile conversion of cysteine and alkyl cysteines to dehydroalanine on protein surfaces: versatile and switchable access to functionalized proteins. J. Am. Chem. Soc. 130, 5052–5053 (2008).
Axup, J. Y. et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc. Natl Acad. Sci. USA 109, 16101–16106 (2012).
Tian, F. et al. A general approach to site-specific antibody drug conjugates. Proc. Natl Acad. Sci. USA 111, 1766–1771 (2014).
Agarwal, P., van der Weijden, J., Sletten, E. M., Rabuka, D. & Bertozzi, C. R. A Pictet–Spengler ligation for protein chemical modification. Proc. Natl Acad. Sci. USA 110, 46–51 (2013).
Drake, P. M. et al. Aldehyde tag coupled with HIPS chemistry enables the production of ADCs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and PK outcomes. Bioconjugate Chem. 25, 1331–1341 (2014).
Li, J. et al. Palladium-triggered deprotection chemistry for protein activation in living cells. Nature Chem. 6, 352–361 (2014).
Li, J., Jia, S. & Chen, P. R. Diels–Alder reaction–triggered bioorthogonal protein decaging in living cells. Nature Chem. Biol. 10, 1003–1005 (2014).
Versteegen, R. M., Rossin, R., ten Hoeve, W., Janssen, H. M. & Robillard, M. S. Click to release: instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem. Int. Ed. 52, 14112–14116 (2013).
Nguyen, D. P. et al. Genetic encoding of photocaged cysteine allows photoactivation of TEV protease in live mammalian cells. J. Am. Chem. Soc. 136, 2240–2243 (2014).
Arbely, E., Torres-Kolbus, J., Deiters, A. & Chin, J. W. Photocontrol of tyrosine phosphorylation in mammalian cells via genetic encoding of photocaged tyrosine. J. Am. Chem. Soc. 134, 11912–11915 (2012).
Liu, W., Brock, A., Chen, S., Chen, S. & Schultz, P. G. Genetic incorporation of unnatural amino acids into proteins in mammalian cells. Nature Methods 4, 239–244 (2007).
Goodman, C. Making sense of nonsense. Nature Chem. Biol. 10, 167–167 (2014).
Acknowledgements
We thank FCT Portugal (FCT Investigator to G.J.L.B.), the EU (Marie-Curie CIG to G.J.L.B. and Marie-Curie IEF to O.B.) and the EPSRC for funding. G.J.L.B. is a Royal Society University Research Fellow. Owing to space limitations, many primary and historical publications have not been cited, in particular in those cases where topical reviews are available.
Author information
Authors and Affiliations
Contributions
N.K. and G.J.L.B. developed the concept, researched and wrote the manuscript. F.P.C. designed and produced the figures, and F.P.C. and O.B. assisted with writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Krall, N., da Cruz, F., Boutureira, O. et al. Site-selective protein-modification chemistry for basic biology and drug development. Nature Chem 8, 103–113 (2016). https://doi.org/10.1038/nchem.2393
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2393
This article is cited by
-
Selenium chemistry for spatio-selective peptide and protein functionalization
Nature Reviews Chemistry (2024)
-
Copper assisted sequence-specific chemical protein conjugation at a single backbone amide
Nature Communications (2023)
-
Derivatization with fatty acids in peptide and protein drug discovery
Nature Reviews Drug Discovery (2023)
-
Site-selective chemical reactions by on-water surface sequential assembly
Nature Communications (2023)
-
SUMO specific peptidase 3 halts pancreatic ductal adenocarcinoma metastasis via deSUMOylating DKC1
Cell Death & Differentiation (2023)