Abstract
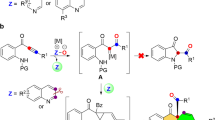
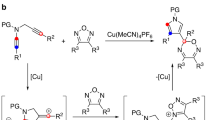
Pyrroles are structurally important heterocycles. However, the synthesis of polysubstituted pyrroles is often challenging. Here, we report a multicomponent, Ti-catalysed formal [2+2+1] reaction of alkynes and diazenes for the oxidative synthesis of penta- and trisubstituted pyrroles: a nitrenoid analogue to classical Pauson–Khand-type syntheses of cyclopentenones. Given the scarcity of early transition-metal redox catalysis, preliminary mechanistic studies are presented. Initial stoichiometric and kinetic studies indicate that the mechanism of this reaction proceeds through a formally TiII/TiIV redox catalytic cycle, in which an azatitanacyclobutene intermediate, resulting from [2+2] alkyne + Ti imido coupling, undergoes a second alkyne insertion followed by reductive elimination to yield pyrrole and a TiII species. The key component for catalytic turnover is the reoxidation of the TiII species to a TiIV imido via the disproportionation of an η2-diazene-TiII complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
02 December 2015
In the original version of this Article published online, the structure of 4n (shown in Table 1) was incorrect. The structure has been corrected in all versions of the Article.
References
Bullock, R. M. Catalysis without Precious Metals (Wiley, 2010).
Mikami, K., Terada, M. & Matsuzawa, H. ‘Asymmetric’ catalysis by lanthanide complexes. Angew. Chem. Int. Ed. 41, 3554–3571 (2002).
Nishiura, M. & Hou, Z. Novel polymerization catalysts and hydride clusters from rare-earth metal dialkyls. Nature Chem. 2, 257–268 (2010).
Dudnik, A. S., Weidner, V. L., Motta, A., Delferro, M. & Marks, T. J. Atom-efficient regioselective 1,2-dearomatization of functionalized pyridines by an earth-abundant organolanthanide catalyst. Nature Chem. 6, 1100–1107 (2014).
Müller, T. E., Hultzsch, K. C., Yus, M., Foubelo, F. & Tada, M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem. Rev. 108, 3795–3892 (2008).
Odom, A. New C–N and C–C bond forming reactions catalyzed by titanium complexes. Dalton Trans. 225–233 (2005).
Basuli, F., Wicker, B., Huffman, J. C. & Mindiola, D. J. Understanding the role of an easy-to-prepare aldimine-alkyne carboamination catalyst, [Ti(NMe2)3(NHMe2)][B(C6F5)4]. J. Orgomet. Chem. 696, 235–243 (2011).
Kulinkovich, O. G. The chemistry of cyclopropanols. Chem. Rev. 103, 2597–2632 (2003).
Hicks, F. A., Kablaoui, N. M. & Buchwald, S. L. Scope of the intramolecular titanocene-catalyzed Pauson–Khand type reaction. J. Am. Chem. Soc. 121, 5881–5898 (1999).
Ozerov, O. V., Patrick, B. O. & Lapido, F. T. Highly regioselective [2+2+2] cycloaddition of alkynes catalyzed by η6-arene complexes of titanium supported by dimethylsilyl-bridged p-tert-butyl calix[4]arene ligand. J. Am. Chem. Soc. 122, 6423–6431 (2000).
Negishi, E., Swanson, D. R., Cederbaum, F. E. & Takahashi, T. Zirconium-promoted bicyclization of enynes. Effects of enyne structure. Tetrahedron Lett. 28, 917–920 (1987).
Al Dulayymi, J. R., Baird, M. S., Bolesov, I. G., Tveresovsky, V. & Rubin, M. A simple and efficient hydrodehalogenation of 1,1-dihalocyclopropanes. Tetrahedron Lett. 37, 8933–8936 (1996).
Nguyen, A. I., Zarkesh, R. A., Lacy, D. C., Thorson, M. K. & Heyduk, A. F. Catalytic nitrene transfer by a zirconium(IV) redox-active ligand complex. Chem. Sci. 2, 166–169 (2011).
Baumann, M., Baxendale, I. R., Ley, S. V. & Nikbin, N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 7, 442–495 (2011).
Vernitskaya, T. V. & Efimov, O. N. Polypyrrole: a conducting polymer; its synthesis, properties, and applications. Russ. Chem. Rev. 66, 443–457 (1997).
Loudet, A. & Burgess, K. BODIPY dyes and their derivatives: syntheses and spectroscopic studies. Chem. Rev. 107, 4891–4932 (2007).
Walsh, C. T., Tsodikova, S. G. & Howard-Jones, A. R. Biological formation of pyrroles: nature's logic and enzymatic machinery. Nat. Prod. Rep. 23, 517–531 (2006).
Estévez, V., Villacampa, M. & Menéndez, J. C. Recent advances in the synthesis of pyrroles by multicomponent reactions. Chem. Soc. Rev. 43, 4633–4657 (2014).
Martin, R., Larsen, C. H., Cuenca, A. & Buchwald, S. L. Cu-catalyzed tandem C–N bond formation for the synthesis of pyrroles and heteroarylpyrroles. Org. Lett. 9, 3379–3382 (2007).
Ramanathan, B., Keith, A. J., Armstrong, D. & Odom, A. L. Pyrrole syntheses based on titanium-catalyzed hydroamination of diynes. Org. Lett. 6, 2957–2960 (2004).
Gulevich, A. V., Dudnik, A. S., Cernyak, N. & Gevorgyan, V. Transition metal-mediated synthesis of monocyclic aromatic heterocycles. Chem. Rev. 113, 3084–3213 (2013).
Yu, S., Xiong, M., Xie, X. & Lui, Y. Insertion of nitriles into zirconocene 1-aza-1,3-diene complexex: chemoselective synthesis of N–H and N-substituted pyrroles. Angew. Chem. Int. Ed. 53, 11596–11599 (2014).
Nakamoto, M. & Tilley, T. D. Reactions of zirconacyclopentadienes with nitrosobenzene. Characterization of zirconacycle intermediates and formation of N-phenylpyrroles. Organometallics 20, 5515–5517 (2001).
Rosenthal, U., Burlakov, V. V., Bach, M. A. & Beweries, T. Five-membered metallacycles of titanium and zirconium—attractive compounds for organometallics chemistry and catalysis. Chem. Soc. Rev. 36, 719–728 (2007).
Liu, W., Jiang, H. & Huang, L. One-pot silver-catalzyed and PIDA-mediated sequential reactions: synthesis of polysubstituted pyrroles directly from alkynoates and amines. Org. Lett. 12, 312–315 (2010).
Blanco-Urgoiti, J., Añorbe, L., Pérez-Serrano, L. & Domínguez, G. The Pauson–Khand reaction, a powerful synthetic tool for the synthesis of complex molecules. Chem. Soc. Rev. 33, 32–42 (2004).
Omae, I. Three characteristic reactions of alkynes with metal compounds in organic synthesis. Appl. Organometal. Chem. 22, 149–166 (2008).
Chopade, P. R. & Louie, J. [2+2+2] cycloaddition reactions catalyzed by transition metals. Adv. Synth. Catal. 348, 2307–2327 (2006).
Wender, P. A. et al. Inspirations from nature. New reactions, therapeutic leads, and drug delivery systems. Pure Appl. Chem. 75, 143–155 (2003).
Hill, J. E., Fanwick, P. E. & Rothwell, I. P. Formation of a terminal aryl-imido compound of titanium by cleavage of the N=N double bond in benzo[c]cinnoline. Inorg. Chem. 30, 1143–1144 (1991).
Tonks, I. A., Meier, J. C. & Bercaw, J. E. Titanium complexes supported by pyridine-bis(phenolate) ligands: active catalysts for intermolecular hydroamination or trimerization of alkynes. Organometallics 32, 3451–3457 (2013).
Vujokvic, N. et al. Imido-alkyne coupling in titanium complexes: new insight into the alkyne hydroamination reaction. Organometallics 26, 5522–5534 (2007).
Lokare, K. S., Ciszewski, J. T. & Odom, A. L. Group-6 imido activation by a ring-strained alkyne. Organometallics 23, 5386–5388 (2004).
Fout, A. R., Kilgore, U. J. & Mindiola, D. J. The recent progression of synthetic strategies to assemble titanium complexes bearing the terminal imide group. Chem. Eur. J. 13, 9428–9440 (2007).
Duchateau, R., Williams, A. J., Gambarotta, S. & Chiang, M. Y. Carbon-carbon double-bond formation in the intermolecular acetonitrile reductive coupling promoted by a mononuclear titanium(II) compound. Preparation and characterization of two titanium(IV) imido derivatives. Inorg. Chem. 30, 4863–4866 (1991).
Gray, S. D., Thorman, J. L., Adamian, V. A., Kadish, K. M. & Woo, L. K. Synthesis, electrochemistry, and imido and transfer reactions of (TTP)Ti(η2-PhN=NPh). Inorg. Chem. 37, 1–4 (1998).
Hill, B. J. E., Profile, R. D., Fanwick, P. E. & Rothwell, Z. P. Synthesis, structure, and reactivity of aryloxo(imido)titanium complexes. Angew. Chem. Int. Ed. Engl. 102, 664–665 (1990).
Adams, N. et al. New titanium imido synthons: syntheses and supramolecular structures. Inorg. Chem. 44, 2882–2894 (2005).
Blake, A. J. et al. Synthesis and imido-group exchange reactions of tert-butylimidotitanium complexes. J. Chem. Soc. Dalton Trans. 1549–1558 (1997).
Vujkovic, N. et al. Insertions into azatitanacyclobutenes: new insights into three-component coupling raections involving imidotitanium intermediates. Organometallics 27, 2518–2528 (2008).
Straub, B. F. & Bergman, R. G. The mechanism of hydroamination of allenes, alkynes, and alkenes catalyzed by cyclopentadienyltitanium–imido complexes: a density functional study. Angew. Chem. Int. Ed. 40, 4632–4635 (2001).
Weitershaus, K. et al. Titanium hydroamination catalysts bearing a 2-aminopyrrolinto spectator ligand: monitoring the individual reaction steps. Dalton Trans. 4586–4602 (2009).
Barnea, E., Majumder, S., Staples, R. J. & Odom, A. L. One step route to 2,3-diaminopyrroles using a titanium-catalyzed four-component coupling. Organometallics 28, 3876–3881 (2009).
Tripepi, G., Young, V. G. Jr & Ellis, J. E. Highly reduced organometallics Part 49. Reaction of hexacarbonyltitanate(2–) with azobenzene. Structural characterization of the first hydroxo-carbonyl of titanium [Ti2(μ-OH)2(CO)8]2−. J. Organomet. Chem. 593–594, 354–360 (2000).
Kaleta, K., Arndt, P., Spannenberg, A. & Rosenthal, U. Unusual bond activation processes in the reaction of group 4 cyclopentadienyl alkyne complexes with azobenzene. Inorg. Chim. Acta 370, 187–190 (2011).
Kaleta, K. et al. Reactions of group 4 metallocene alkyne complexes with azobenzene: formation of diazametallacyclopropanes and N=N bond activation. Organometallics 29, 2604–2609 (2010).
Retbøll, M. & Jørgensen, K. A. MO explanation of the structures of azo-transition metal complexes. Inorg. Chem. 33, 6403–6405 (1994).
Goetze, B., Knizek, J., Noth, H. & Schnick, W. 1,2-Bis(trimethylsilyl)hydrazido titanium complexes. Eur. J. Inorg. Chem. 1849–1854 (2000).
Munhá, R. F., Zarkesh, R. A. & Heyduk, A. F. Group transfer reactions of d0 transition metal complexes: redox-active ligands provide a mechanism for expanded reactivity. Dalton Trans. 42, 3751–3766 (2013).
Zarkesh, R. A., Ziller, J. W. & Heyduk, A. F. Four-electron oxidative formation of aryl diazenes using a tantalum redox-active ligand complex. Angew. Chem. Int. Ed. 47, 4715–4718 (2008).
Mankad, N. P., Müller, P. & Peters, J. C. Catalytic N–N coupling of aryl azides to yield azoarenes via trigonal bipyramid iron–nitrene intermediates. J. Am. Chem. Soc. 132, 4083–4085 (2010).
Mansuy, D., Battioni, P. & Mahy, J. P. Isolation of an iron–nitrene complex from dioxygen- and iron poryhrin-dependent oxidation of a hydrazine. J. Am. Chem. Soc. 104, 4487–4489 (1982).
Gräbe, K., Pohlki, F. & Doye, S. Neutral Ti complexes as catalysts for the hydroamination of alkynes and alkenes: do the labile ligands change the catalytic activity? Eur. J. Org. Chem. 28, 4815–4823 (2008).
Hicks, F. & Buchwald, S. L. Highly-enantioselective catalytic Pauson–Khand type formation of bicyclic cyclopentenones. J. Am. Chem. Soc. 118, 11688–11689 (1996).
Yim, J. C. H., Bexrud, J. A., Ayinla, R. O., Leitch, D. C. & Schafer, L. L. Bis(amidate)bis(amido) titanium complex: a regioselective intermolecular alkyne hydroamination catalyst. J. Org. Chem. 79, 2015–2028 (2014).
Acknowledgements
Financial support was provided by the University of Minnesota (start-up funds). Equipment purchases for the Chemistry Department NMR facility were supported by a grant from the National Institutes of Health (S10OD011952) with matching funds from the University of Minnesota. The Bruker-AXS D8 Venture diffractometer was purchased through a grant from NSF/MRI (1224900) and the University of Minnesota.
Author information
Authors and Affiliations
Contributions
Z.W.G. and I.A.T. conceived and designed the experiments. Z.W.G. and R.J.H. performed the experiments and analysed the data. I.A.T. wrote the manuscript. All authors contributed to revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4630 kb)
Supplementary information
Crystallographic data for compound 7 (CIF 23 kb)
Rights and permissions
About this article
Cite this article
Gilbert, Z., Hue, R. & Tonks, I. Catalytic formal [2+2+1] synthesis of pyrroles from alkynes and diazenes via TiII/TiIV redox catalysis. Nature Chem 8, 63–68 (2016). https://doi.org/10.1038/nchem.2386
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2386
This article is cited by
-
Probing N-heterocyclic olefin as ancillary ligand in scandium-mediated \(\hbox {CO}_2\) to CO conversion
Theoretical Chemistry Accounts (2020)
-
Synthesis of pyrroles via ruthenium-catalyzed nitrogen-transfer [2 + 2 + 1] cycloaddition of α,ω-diynes using sulfoximines as nitrene surrogates
Communications Chemistry (2018)
-
Modern applications of low-valent early transition metals in synthesis and catalysis
Nature Reviews Chemistry (2018)
-
Quantifying ligand effects in high-oxidation-state metal catalysis
Nature Chemistry (2017)