Abstract
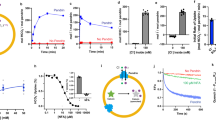
Transmembrane anion transporters (anionophores) have potential for new modes of biological activity, including therapeutic applications. In particular they might replace the activity of defective anion channels in conditions such as cystic fibrosis. However, data on the biological effects of anionophores are scarce, and it remains uncertain whether such molecules are fundamentally toxic. Here, we report a biological study of an extensive series of powerful anion carriers. Fifteen anionophores were assayed in single cells by monitoring anion transport in real time through fluorescence emission from halide-sensitive yellow fluorescent protein. A bis-(p-nitrophenyl)ureidodecalin shows especially promising activity, including deliverability, potency and persistence. Electrophysiological tests show strong effects in epithelia, close to those of natural anion channels. Toxicity assays yield negative results in three cell lines, suggesting that promotion of anion transport may not be deleterious to cells. We therefore conclude that synthetic anion carriers are realistic candidates for further investigation as treatments for cystic fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sessler, J. L. & Allen, W. E. Anion carriers: new tools for crossing membranes. Chemtech. 29, 16–24 (1999).
Davis, A. P., Sheppard, D. N. & Smith, B. D. Development of synthetic membrane transporters for anions. Chem. Soc. Rev. 36, 348–357 (2007).
Davis, J. T., Okunola, O. & Quesada, R. Recent advances in the transmembrane transport of anions. Chem. Soc. Rev. 39, 3843–3862 (2010).
Busschaert, N. & Gale, P. A. Small-molecule lipid-bilayer anion transporters for biological applications. Angew. Chem. Int. Ed. 52, 1374–1382 (2013).
Pressman, B. C. Biological applications of ionophores. Ann. Rev. Biochem. 45, 501–530 (1976).
Dutton, C. J., Banks, B. J. & Cooper, C. B. Polyether ionophores. Nat. Prod. Rep. 12, 165–181 (1995).
Vargas Jentzsch, A., Hennig, A., Mareda, J. & Matile, S. Synthetic ion transporters that work with anion–π interactions, halogen bonds, and anion–macrodipole interactions. Acc. Chem. Res. 46, 2791–2800 (2013).
Gale, P. A., Perez-Tomas, R. & Quesada, R. Anion transporters and biological systems. Acc. Chem. Res. 46, 2801–2813 (2013).
Gokel, G. W. & Negin, S. Synthetic ion channels: from pores to biological applications. Acc. Chem. Res. 46, 2824–2833 (2013).
Valkenier, H. & Davis, A. P. Making a match for valinomycin: steroidal scaffolds in the design of electroneutral, electrogenic anion carriers. Acc. Chem. Res. 46, 2898–2909 (2013).
Wallace, D. P. et al. A synthetic channel-forming peptide induces Cl− secretion: modulation by Ca2+-dependent K+ channels. Biochim. Biophys. Acta 1464, 69–82 (2000).
Ashcroft, F. M. Ion Channels and Disease (Academic Press, 2000).
Stoltz, D. A., Meyerholz, D. K. & Welsh, M. J. Origins of cystic fibrosis lung disease. N. Engl. J. Med. 372, 351–362 (2015).
Jiang, C. W. et al. Partial correction of defective Cl− secretion in cystic fibrosis epithelial cells by an analog of squalamine. Am. J. Physiol. Lung Cell Mol. Physiol. 281, L1164–L1172 (2001).
Broughman, J. R. et al. Distinct structural elements that direct solution aggregation and membrane assembly in the channel-forming peptide M2GlyR. Biochemistry 41, 7350–7358 (2002).
Broughman, J. R. et al. Channel-forming peptide modulates transepithelial electrical conductance and solute permeability. Am. J. Physiol. Cell Physiol. 286, C1312–C1323 (2004).
Pajewski, R. et al. A synthetic, chloride-selective channel that alters chloride transport in epithelial cells. Chem. Commun. 329–331 (2006).
Koulov, A. V. et al. Chloride transport across vesicle and cell membranes by steroid-based receptors. Angew. Chem. Int. Ed. 42, 4931–4933 (2003).
Shen, B., Li, X., Wang, F., Yao, X. Q. & Yang, D. A synthetic chloride channel restores chloride conductance in human cystic fibrosis epithelial cells. PLoS ONE 7, e34694 (2012).
Sidorov, V. et al. Ion channel formation from a calix 4 arene amide that binds HCl. J. Am. Chem. Soc. 124, 2267–2278 (2002).
Sessler, J. L. et al. Synthesis, anion-binding properties, and in vitro anticancer activity of prodigiosin analogues. Angew. Chem. Int. Ed. 44, 5989–5992 (2005).
de Grenu, B. D. et al. Synthetic prodiginine obatoclax (GX15–070) and related analogues: anion binding, transmembrane transport, and cytotoxicity properties. Chem. Eur. J. 17, 14074–14083 (2011).
Busschaert, N. et al. Structure–activity relationships in tripodal transmembrane anion transporters: the effect of fluorination. J. Am. Chem. Soc. 133, 14136–14148 (2011).
Moore, S. J. et al. Chloride, carboxylate and carbonate transport by ortho-phenylenediamine-based bisureas. Chem. Sci. 4, 103–117 (2013).
Ko, S. K. et al. Synthetic ion transporters can induce apoptosis by facilitating chloride anion transport into cells. Nature Chem. 6, 885–892 (2014).
Davis, J. T. Anion binding and transport by prodigiosin and its analogs. Top. Heterocyclic Chem. 24, 145–176 (2010).
Brotherhood, P. R. & Davis, A. P. Steroid-based anion receptors and transporters. Chem. Soc. Rev. 39, 3633–3647 (2010).
McNally, B. A. et al. Structure–activity relationships in cholapod anion carriers: enhanced transmembrane chloride transport through substituent tuning. Chem. Eur. J. 14, 9599–9606 (2008).
Edwards, S. J., Valkenier, H., Busschaert, N., Gale, P. A. & Davis, A. P. High affinity anion binding by steroidal squaramide receptors. Angew. Chem. Int. Ed. 54, 4592–4596 (2015).
Hussain, S., Brotherhood, P. R., Judd, L. W. & Davis, A. P. Diaxial diureido decalins as compact, efficient, and tunable anion transporters. J. Am. Chem. Soc. 133, 1614–1617 (2011).
Valkenier, H. et al. Preorganized bis-thioureas as powerful anion carriers: chloride transport by single molecules in large unilamellar vesicles. J. Am. Chem. Soc. 136, 12507–12512 (2014).
Cooper, J. A., Street, S. T. G. & Davis, A. P. A flexible solution to anion transport: powerful anionophores based on a cyclohexane scaffold. Angew. Chem. Int. Ed. 53, 5609–5613 (2014).
McNally, B. A., Koulov, A. V., Smith, B. D., Joos, J. B. & Davis, A. P. A fluorescent assay for chloride transport; identification of a synthetic anionophore with improved activity. Chem. Commun. 1087–1089 (2005).
Clare, J. P. et al. Substrate discrimination by cholapod anion receptors: geometric effects and the ‘affinity-selectivity principle’. J. Am. Chem. Soc. 127, 10739–10746 (2005).
Li, X., Shen, B., Yao, X. Q. & Yang, D. A small synthetic molecule forms chloride channels to mediate chloride transport across cell membranes. J. Am. Chem. Soc. 129, 7264–7265 (2007).
Verkman, A. S. & Galietta, L. J. V. Chloride channels as drug targets. Nature Rev. Drug Discov. 8, 153–171 (2009).
Galietta, L. J. V., Haggie, P. M. & Verkman, A. S. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 499, 220–224 (2001).
Sheppard, D. N., Carson, M. R., Ostedgaard, L. S., Denning, G. M. & Welsh, M. J. Expression of cystic fibrosis transmembrane conductance regulator in a model epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 266, L405–L413 (1994).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 46, 3–26 (2001).
Cutting, G. R. Cystic fibrosis genetics: from molecular understanding to clinical application. Nature Rev. Genet. 16, 45–56 (2015).
Alton, E. W. F. W. et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet. Respir. Med. 3, 684–691 (2015).
Ramsey, B. W. et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 365, 1663–1672 (2011).
Wainwright, C. E. et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N. Engl. J. Med. 373, 220–231 (2015).
Mall, M. A. & Galietta, L. J. V. Targeting ion channels in cystic fibrosis. J. Cyst. Fibros. 14, 561–570 (2015).
McLachlan, G. et al. Optimizing aerosol gene delivery and expression in the ovine lung. Mol. Ther. 15, 348–354 (2007).
Galietta, L. V. J., Jayaraman, S. & Verkman, A. S. Cell-based assay for high-throughput quantitative screening of CFTR chloride transport agonists. Am. J. Physiol. Cell Physiol. 281, C1734–C1742 (2001).
Li, H., Findlay, I. A. & Sheppard, D. N. The relationship between cell proliferation, Cl− secretion, and renal cyst growth: a study using CFTR inhibitors. Kidney Int. 66, 1926–1938 (2004).
Acknowledgements
The authors thank A.S. Verkman for the gift of FRT cells expressing YFP-H148Q/I152L, the Wolfson Bioimaging Facility (University of Bristol) and M.A. Jepson and A.D. Leard for help and advice. This work was supported by the Engineering and Physical Sciences Research Council (grants nos. EP/F03623X/1 and EP/J00961X/1). O.J. thanks K. Rissanen for a postdoctoral position through the Academy of Finland (grant no. 265328) and the University of Jyväskylä for an international mobility grant.
Author information
Authors and Affiliations
Contributions
A.P.D., D.N.S. and H.L. conceived and designed the experiments. H.L., H.V., L.W.J., P.R.B., S.H., J.A.C., O.J. and H.A.S. performed the research. A.P.D., D.N.S, H.L. and H.V. analysed the data and co-wrote the manuscript. A.P.D. and D.N.S. directed the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2125 kb)
Supplementary information
Crystallographic data for compound 13 (CIF 851 kb)
Rights and permissions
About this article
Cite this article
Li, H., Valkenier, H., Judd, L. et al. Efficient, non-toxic anion transport by synthetic carriers in cells and epithelia. Nature Chem 8, 24–32 (2016). https://doi.org/10.1038/nchem.2384
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2384
This article is cited by
-
Measuring anion binding at biomembrane interfaces
Nature Communications (2022)
-
Near quantitative synthesis of urea macrocycles enabled by bulky N-substituent
Nature Communications (2021)
-
Engineering of stimuli-responsive lipid-bilayer membranes using supramolecular systems
Nature Reviews Chemistry (2020)
-
Ionophore constructed from non-covalent assembly of a G-quadruplex and liponucleoside transports K+-ion across biological membranes
Nature Communications (2020)
-
Pore-forming small molecules offer a promising way to tackle cystic fibrosis
Nature (2019)