Abstract
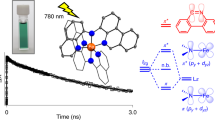
Solar energy conversion in photovoltaics or photocatalysis involves light harvesting, or sensitization, of a semiconductor or catalyst as a first step. Rare elements are frequently used for this purpose, but they are obviously not ideal for large-scale implementation. Great efforts have been made to replace the widely used ruthenium with more abundant analogues like iron, but without much success due to the very short-lived excited states of the resulting iron complexes. Here, we describe the development of an iron–nitrogen–heterocyclic-carbene sensitizer with an excited-state lifetime that is nearly a thousand-fold longer than that of traditional iron polypyridyl complexes. By the use of electron paramagnetic resonance, transient absorption spectroscopy, transient terahertz spectroscopy and quantum chemical calculations, we show that the iron complex generates photoelectrons in the conduction band of titanium dioxide with a quantum yield of 92% from the 3MLCT (metal-to-ligand charge transfer) state. These results open up possibilities to develop solar energy-converting materials based on abundant elements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hagfeldt, A. & Grätzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 95, 49–68 (1995).
Anderson, N. A. & Lian, T. Ultrafast electron transfer at the molecule-semiconductor nanoparticle interface. Annu. Rev. Phys. Chem. 56, 491–519 (2005).
Hagfeldt, A., Boschloo, G., Sun, L., Kloo, L. & Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 110h, 6595–6663 (2010).
Youngblood, W. J., Lee, S. A., Maeda, K. & Mallouk, T. E. Visible light water splitting using dye-sensitized oxide semiconductors. Acc. Chem. Res. 42, 1966–1973 (2009).
Sun, L., Li, F. & Yu, Z. Recent advances in dye-sensitized photoelectrochemical cells for solar hydrogen production based on molecular components. Energy Environ. Sci. 8, 760–775 (2015).
Park, H., Park, Y., Kim, W. & Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Rev. 15, 1–20 (2013).
Li, L. & Diau, E. W. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 42, 291–304 (2013).
Grätzel, M. The light and shade of perovskite solar cells. Nature Mater. 13, 838–842 (2014).
Campagna, S., Puntoriero, F., Nastasi, F., Bergamini, G. & Balzani, V. Photochemistry and photophysics of coordination compounds. An extended view. Coord. Chem. Rev. 280, 117–214 (2007).
Creutz, C., Chou, M., Netzel, T. L., Okumura, M. & Sutin, N. Lifetimes, spectra, and quenching of the excited states of polypyridine complexes of iron(II), ruthenium(II), and osmium(II). J. Am. Chem. Soc. 102, 1309–1319 (1980).
McCusker, J. K. et al. Subpicosecond 1MLCT 5T2 intersystem crossing of low-spin polypyridyl ferrous complexes. J. Am. Chem. Soc. 115, 298–307 (1993).
McCusker, J. K., Rheingold, A. L. & Hendrickson, D. N. Variable-temperature studies of laser-initiated 5T2–1A1 intersystem crossing in spin-crossover complexes: empirical correlations between activation parameters and ligand structure in a series of polypyridyl ferrous complexes. Inorg. Chem. 35, 2100–2112 (1996).
Monat, J. E. & McCusker, J. K. Femtosecond excited-state dynamics of an iron(II) polypyridyl solar cell sensitizer model. J. Am. Chem. Soc. 122, 4092–4097 (2000).
Juban, E. A., Smeigh, A. L., Monat, J. E. & McCusker, J. K. Ultrafast dynamics of ligand-field excited states. Coord. Chem. Rev. 250, 1783–1791 (2006).
Smeigh, A. L., Creelman, M., Mathies, R. A. & McCusker, J. K. Femtosecond time-resolved optical and Raman spectroscopy of photoinduced spin crossover: temporal resolution of low- to-high spin optical switching. J. Am. Chem. Soc. 130, 14105–14107 (2008).
Cannizzo, A. et al. Light-induced spin crossover in Fe(II)-based complexes. The full photocycle unraveled by ultrafast optical and X-ray spectroscopies. Coord. Chem. Rev. 254, 2677–2686 (2010).
Jamula, L. L., Brown, A. M., Guo, D. & McCusker, J. K. Synthesis and characterization of a high-symmetry ferrous polypyridyl complex: approaching the 5T2/3T11 crossing point for Fe. Inorg. Chem. 53, 15–17 (2014).
Zhang, W. et al. Tracking excited-state charge and spin dynamics in iron coordination complexes. Nature 509, 345–348 (2014).
Schnadt, J. et al. Experimental evidence for sub-3-fs charge transfer from an aromatic adsorbate to a semiconductor. Nature 418, 620–623 (2002).
Persson, P., Lundqvist, M. J., Ernstorfer, R., Goddard, W. A. & Willig, F. Quantum chemical calculations of the influence of anchor-cum-spacer groups on femtosecond electron transfer times in dye-sensitized semiconductor nanocrystals. J. Chem. Theory Comput. 2, 441–451 (2006).
Benkö, G., Kallioinen, J., Korppi-Tommola, J. E. I., Yartsev, A. P. & Sundström, V. Photoinduced ultrafast dye-to-semiconductor electron injection from nonthermalized and thermalized donor states. J. Am. Chem. Soc. 124, 489–493 (2002).
Ferrere, S. & Gregg, B. A. Photosensitization of TiO2 by band selective electron injection from ultra-short-lived excited states. J. Am. Chem. Soc. 120, 843–844 (1998).
Ferrere, S. New photosensitizers based upon [Fe(L)2(CN)2] and [Fe(L)3] (L= substituted 2,2-bipyridine): yields for the photosensitization of TiO2 and effects on the band selectivity. Chem. Mater. 12, 1083–1089 (2000).
Ferrere, S. New photosensitizers based upon [FeII(L)2(CN)2] and [FeIIL3], where L is substituted 2,2′-bipyridine. Inorg. Chim. Acta 329, 79–92 (2002).
Yang, M., Thompson, D. W. & Meyer, G. J. Dual pathways for TiO2 sensitization by Na2[Fe(bpy)(CN)4]. Inorg. Chem. 39, 3738–3739 (2000).
Yang, M., Thompson, D. W. & Meyer, G. J. Charge-transfer studies of iron cyano compounds bound to nanocrystalline TiO2 surfaces. Inorg. Chem. 41, 1254–1262 (2002).
Bowman, D. N., Blew, J. H., Tsuchiya, T. & Jakubikova, E. Elucidating band-selective sensitization in iron(II) polypyridine–TiO2 assemblies. Inorg. Chem. 52, 8621–8628 (2013).
Bowman, D. N., Mukherjee, S., Barnes, L. J. & Jakubikova, E. Linker dependence of interfacial electron transfer rates in Fe(II)–polypyridine sensitized solar cells. J. Phys. Condens. Matter 27, 134205 (2015).
Dixon, I. M., Alary, F., Boggio-Pasqua, M. & Heully, J.-L. The (N4C2)2– donor set as promising motif for bis(tridentate) iron(II) photoactive compounds. Inorg. Chem. 52, 13369–13374 (2013).
Mukherjee, S., Bowman, D. N. & Jakubikova, E. Cyclometalated Fe(II) complexes as sensitizers in dye-sensitized solar cells. Inorg. Chem. 54, 560–569 (2015).
Duchanois, T. et al. Heteroleptic pyridyl–carbene iron complexes with tuneable electronic properties. Eur. J. Inorg. Chem. 2014, 3747–3753 (2014).
Liu, Y. et al. Towards longer-lived metal-to-ligand charge transfer states of iron(II) complexes an N-heterocyclic carbene approach. Chem. Commun. 49, 6412–6414 (2013).
Fredin, L. A. et al. Exceptional excited-state lifetime of an iron(II) N-heterocyclic carbene complex explained. J. Phys. Chem. Lett. 5, 2066–2071 (2014).
Liu, Y. et al. A heteroleptic ferrous complex with mesoionic bis(1,2,3-triazol-5-ylidene) ligands taming the MLCT excited state of iron(II). Chem. Eur. J. 21, 3628–3639 (2015).
Duchanois, T. et al. An iron-based photosensitizer with extended excited-state lifetime photophysical and photovoltaic properties. Eur. J. Inorg. Chem. 2015, 2469–2477 (2015).
Huynh, H. V. & Frison, G. Electronic structural trends in divalent carbon compounds. J. Org. Chem. 78, 328–338 (2013).
Galynska, M. & Persson, P. Emerging polymorphism in nanostructured TiO2: quantum chemical comparison of anatase, rutile, and brookite clusters. Int. J. Quantum Chem. 113, 2611–2620 (2013).
de Angelis, F., Valentin, C. D., Fantacci, S., Vittadini, A. & Selloni, A. Theoretical studies on anatase and less common TiO2 phases: bulk, surfaces, and nanomaterials. Chem. Rev. 114, 9708–9753 (2014).
Huang, J. et al. Highly efficient ultrafast electron injection from the singlet MLCT excited state of copper(I) diimine complexes to TiO2 nanoparticles. Angew. Chem. Int. Ed. 51, 12711–12715 (2012).
Abragam, A. & Bleaney, B. in Electron Paramagnetic Resonance of Transition Ions (Oxford Univ. Press, 1970).
Pilbrow, J. R. Transition Ion Electron Paramagnetic Resonance (Clarendon, 1990).
Xiong, L.-B., Li, J.-L., Yang, B. & Yu, Y. Ti3+ in the surface of titanium dioxide: generation, properties and photocatalytic application. J. Nanomater. 2012, 831524 (2012).
Němec, H. et al. Influence of the electron–cation interaction on electron mobility in dye-sensitized ZnO and TiO2 nanocrystals. A study using ultrafast terahertz spectroscopy. Phys. Rev. Lett. 104, 197401 (2010).
Němec, H., Kužel, P. & Sundström, V. Charge transport in nanostructured materials for solar energy conversion studied by time-resolved terahertz spectroscopy. J. Photochem. Photobiol. A 215, 123–139 (2010).
Katoh, R. & Furube, A. Electron injection efficiency in dye-sensitized solar cells. J. Photochem. Photobiol. C 20, 1–16 (2014).
Ponseca, C. S. et al. Ultrafast terahertz photoconductivity of bulk heterojunction materials reveals high carrier mobility up to nanosecond time scale. J. Am. Chem. Soc. 134, 11836–11839 (2012).
Hara, K. et al. Highly efficient photon-to-electron conversion with mercurochrome-sensitized nanoporous oxide semiconductor solar cells. Sol. Energ. Mater. Sol. Cells 64, 115–134 (2000).
Frisch, M. J. et al. Gaussian09 Revision D.01 (Gaussian, 2009).
Perdew, J. P., Ernzerhof, M. & Burke, K. Generalized gradient approximation made simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 78, 1396 (1997).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158 (1999).
Acknowledgements
The authors thank E. Thyrhaug for help with steady-state absorption measurements, H. Němec for providing the THz photoconductivity kinetics of RuN3 on TiO2 and E. Unger for valuable discussions. This work was supported by the Crafoord Foundation, the Swedish Research Council (VR), the Knut and Alice Wallenberg (KAW) Foundation and the Swedish Energy Agency. P.P. acknowledges support from the Swedish National Supercomputing Centre and the Lund University Intensive Computation Application Research Center supercomputing facilities. The European Research Council is acknowledged for an Advanced Investigator Grant to V.S. (226136-VISCHEM).
Author information
Authors and Affiliations
Contributions
T.H., Y.L., P.C., K.K., V.S. and K.W. conceived and designed the experiments. Y.L. and O.G. performed the synthesis. Samples were prepared by Y.L., O.G., H.M. and P.C. R.L. carried out the electrochemical measurements. R.W. performed the electron microscopy and energy-dispersive X-ray spectroscopy. L.F. made the quantum chemical calculations and the data were analysed by L.F. and P.P. Y.L. and P.H. performed the electron paramagnetic resonance experiments and the data were analysed by P.H. and S.S. T.H., Y.L., P.C., K.K. and H.M. carried out the transient absorption spectroscopy experiments and the data were analysed by T.H. C.P. conducted the transient terahertz experiments and T.H. analysed the data. T.H., Y.L., V.S. and K.W. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1409 kb)
Rights and permissions
About this article
Cite this article
Harlang, T., Liu, Y., Gordivska, O. et al. Iron sensitizer converts light to electrons with 92% yield. Nature Chem 7, 883–889 (2015). https://doi.org/10.1038/nchem.2365
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2365
This article is cited by
-
Synergistic Excited State Involved Catalytic Reduction of (NH3-trz)[Fe(dipic)2] Complex by SnO2/TiO2 Nanocomposite
Journal of Inorganic and Organometallic Polymers and Materials (2022)
-
Resonant X-ray photo-oxidation of light-harvesting iron (II/III) N-heterocyclic carbene complexes
Scientific Reports (2021)
-
Delayed fluorescence from a zirconium(iv) photosensitizer with ligand-to-metal charge-transfer excited states
Nature Chemistry (2020)
-
Vibrational wavepacket dynamics in Fe carbene photosensitizer determined with femtosecond X-ray emission and scattering
Nature Communications (2020)
-
Iron(ii) coordination complexes with panchromatic absorption and nanosecond charge-transfer excited state lifetimes
Nature Chemistry (2019)