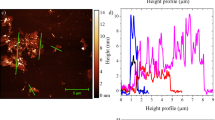
Abstract
Graphene has shown much promise as an organic electronic material but, despite recent achievements in the production of few-layer graphene, the quantitative exfoliation of graphite into pristine single-layer graphene has remained one of the main challenges in developing practical devices. Recently, reduced graphene oxide has been recognized as a non-feasible alternative to graphene owing to variable defect types and levels, and attention is turning towards reliable methods for the high-throughput exfoliation of graphite. Here we report that microwave irradiation of graphite suspended in molecularly engineered oligomeric ionic liquids allows for ultrahigh-efficiency exfoliation (93% yield) with a high selectivity (95%) towards ‘single-layer’ graphene (that is, with thicknesses <1 nm) in a short processing time (30 minutes). The isolated graphene sheets show negligible structural deterioration. They are also readily redispersible in oligomeric ionic liquids up to ~100 mg ml–1, and form physical gels in which an anisotropic orientation of graphene sheets, once induced by a magnetic field, is maintained.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Geim, A. K. & Novoselov, K. S. The rise of graphene. Nature Mater. 6, 183–191 (2007).
Geim, A. K. Graphene: status and prospects. Science 324, 1530–1534 (2009).
Novoselov, K. S. et al. A roadmap for graphene. Nature 490, 192–200 (2012).
Mattevi, C., Kim, H. & Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 21, 3324–3334 (2011).
Nicolosi, V., Chhowalla, M., Kanatzidis, M. G., Strano, M. S. & Coleman, J. N. Liquid exfoliation of layered materials. Science 340, 1226419 (2013).
Ciesielski, A. & Samorì, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 43, 381–398 (2014).
Hernandez, Y. et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nature Nanotech. 3, 563–568 (2008).
Lotya, M. et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 131, 3611–3620 (2009).
Coleman, J. N. Liquid-phase exfoliation of nanotubes and graphene. Adv. Funct. Mater. 19, 3680–3695 (2009).
Nuvoli, D. et al. High concentration few-layer graphene sheets obtained by liquid phase exfoliation of graphite in ionic liquid. J. Mater. Chem. 21, 3428–3431 (2011).
Sampath, S. et al. Direct exfoliation of graphite to graphene in aqueous media with diazaperopyrenium dications. Adv. Mater. 25, 2740–2745 (2013).
Liang, Y. T. & Hersam, M. C. Highly concentrated graphene solutions via polymer enhanced solvent exfoliation and iterative solvent exchange. J. Am. Chem. Soc. 132, 17661–17663 (2010).
Wang, X. et al. Direct exfoliation of natural graphite into micrometre size few layers graphene sheets using ionic liquids. Chem. Commun. 46, 4487–4489 (2010).
Ciesielski, A. et al. Harnessing the liquid-phase exfoliation of graphene using aliphatic compounds: a supramolecular approach. Angew. Chem. Int. Ed. 53, 10355–10361 (2014).
Paton, K. R. et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nature Mater. 13, 624–630 (2014).
Zhao, W. et al. Preparation of graphene by exfoliation of graphite using wet ball milling. J. Mater. Chem. 20, 5817–5819 (2010).
Rangappa, D. et al. Rapid and direct conversion of graphite crystals into high-yielding, good-quality graphene by supercritical fluid exfoliation. Chem. Eur. J. 16, 6488–6494 (2010).
Shih, C. J. et al. Bi- and trilayer graphene solutions. Nature Nanotech. 6, 439–445 (2011).
Wei, T., Fan, Z., Luo, G., Zheng, C. & Xie, D. A rapid and efficient method to prepare exfoliated graphite by microwave irradiation. Carbon 47, 337–339 (2008).
Kovtyukhova, N. I. et al. Non-oxidative intercalation and exfoliation of graphite by Brønsted acids. Nature Chem. 6, 957–963 (2014).
Parvez, K. et al. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 136, 6083–6091 (2014).
Zhu, Y. et al. Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 22, 3906–3924 (2010).
Dreyer, D. R., Park, S., Bielawski, C. W. & Ruoff, R. S. The chemistry of graphene oxide. Chem. Soc. Rev. 39, 228–240 (2010).
Zhu, Y. et al. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 48, 2118–2122 (2010).
Bianco, A. et al. All in the graphene family—a recommended nomenclature for two-dimensional carbon materials. Carbon 65, 1–6 (2013).
Wick, P. et al. Classification framework for graphene-based materials. Angew. Chem. Int. Ed. 53, 7714–7718 (2014).
Qin, F. & Brosseau, C. A review and analysis of microwave absorption in polymer composites filled with carbonaceous particles. J. Appl. Phys. 111, 061301 (2012).
Wang, C. et al. The electromagnetic property of chemically reduced graphene oxide and its application as microwave absorbing material. Appl. Phys. Lett. 98, 072906 (2011).
Tang, J., Radosz, M. & Shen, Y. Poly(ionic liquid)s as optically transparent microwave-absorbing materials. Macromolecules 41, 493–496 (2008).
Robinson, J. et al. Understanding microwave heating effects in single mode type cavities—theory and experiment. Phys. Chem. Chem. Phys. 12, 4750–4758 (2010).
Fukushima, T. et al. Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 300, 2072–2074 (2003).
Lee, J. & Aida, T. ‘Bucky gels’ for tailoring electroactive materials and devices the composites of carbon materials with ionic liquids. Chem. Commun. 47, 6757–6762 (2011).
Ma, J. C. & Dougherty, D. A. The cation−π interaction. Chem. Rev. 97, 1303–1324 (1997).
Jia, X. et al. Graphene edges: a review of their fabrication and characterization. Nanoscale 3, 86–95 (2011).
Shim, J. et al. Water-gated charge doping of graphene induced by mica substrates. Nano Lett. 12, 648–654 (2012).
Ochedowski, O., Bussmann, B. K. & Schleberger, M. Graphene on mica—intercalated water trapped for life. Sci. Rep. 4, 6003 (2014).
Ferrari, A. C. et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 97, 187401 (2006).
Lucchese, M. M. et al. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 48, 1592–1597 (2010).
Bae, S. et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nature Nanotech. 5, 574–578 (2010).
Fasting, C. et al. Multivalency as a chemical organization and action principle. Angew. Chem. Int. Ed. 51, 10472–10498 (2012).
Vadahanambi, S., Jung, J. H., Kumar, R., Kim, H. J. & Oh, I. K. An ionic liquid-assisted method for splitting carbon nanotubes to produce graphene nano-ribbons by microwave radiation. Carbon 53, 391–398 (2013).
Rüdorff, W. & Rüdorff, G. Zur Konstitution des Kohlenstoff. Mononuorids. Z. Anorg. Allg. Chem. 253, 281–296 (1947).
Mallouk, T. & Bartlett, N. Reversible intercalation of graphite by fluoride: a new bifluoride, C12HF2, and graphite CxF (5 > x > 2). J. Chem. Soc. Chem. Commun. 103–105 (1983).
Behabtu, N. et al. Spontaneous high-concentration dispersions and liquid crystals of graphene. Nature Nanotech. 5, 406–411 (2010).
Alzari, V. et al. Graphene-containing thermoresponsive nanocomposite hydrogels of poly(N-isopropylacrylamide) prepared by frontal polymerization. J. Mater. Chem. 21, 8727–8733 (2011).
Stamenov, P. & Coey, J. M. D. Magnetic susceptibility of carbon—experiment and theory. J. Magnet. Magn. Mater. 290–291, 279–285 (2005).
Wu, L. et al. Magnetically induced anisotropic orientation of graphene oxide locked by in situ hydrogelation. ACS Nano 8, 4640–4649 (2014).
Fuller, J., Carlin, R. T., De Long, H. C. & Haworth, D. Structure of 1-ethyl-3-methylimidazolium hexafluorophosphate: model for room temperature molten salts. J. Chem. Soc. Chem. Commun. 299–300 (1994).
Wilkes, J. S. & Zaworotko, M. J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun. 965–967 (1992).
Acknowledgements
We acknowledge the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Specially Promoted Research on the Physically Perturbed Assembly for Tailoring High-Performance Soft Materials with Controlled Macroscopic Structural Anisotropy (25000005) and the JSPS FIRST Program for Innovative Basic Research Toward the Creation of a High-performance Battery. We thank E. Silver for generous discussion. SEM, TEM and XPS were conducted at the Research Hub for Advanced Nano Characterization, The University of Tokyo, supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan. We also acknowledge the ImPACT Program of the Council for Science, Technology and Innovation (Cabinet Office, Government of Japan).
Author information
Authors and Affiliations
Contributions
M.M. and Y.S. designed and performed all the experiments. C.P., T.F. and T.A. co-designed the experiments. M.M. and T.A. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1332 kb)
Rights and permissions
About this article
Cite this article
Matsumoto, M., Saito, Y., Park, C. et al. Ultrahigh-throughput exfoliation of graphite into pristine ‘single-layer’ graphene using microwaves and molecularly engineered ionic liquids. Nature Chem 7, 730–736 (2015). https://doi.org/10.1038/nchem.2315
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2315
This article is cited by
-
Review of the role of ionic liquids in two-dimensional materials
Frontiers of Physics (2023)
-
Study on the exfoliation mechanism of graphene nanoplatelets in the polypropylene/graphene nanoplatelets composites under the elongational flow generated by convergent-divergent channels
Journal of Materials Science (2022)
-
Dielectric control of ultralight hollow porous carbon spheres and excellent microwave absorbing properties
Journal of Materials Science (2021)
-
Two-Dimensional Black Phosphorus Nanomaterials: Emerging Advances in Electrochemical Energy Storage Science
Nano-Micro Letters (2020)
-
Recent Advances in Two-dimensional Materials for Electrochemical Energy Storage and Conversion
Chemical Research in Chinese Universities (2020)