Abstract
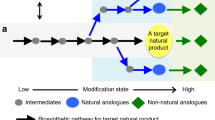
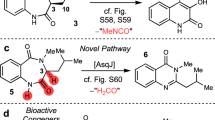
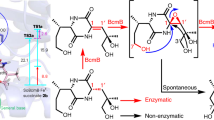
The structural complexity and diversity of natural products make them attractive sources for potential drug discovery, with their characteristics being derived from the multi-step combination of enzymatic and non-enzymatic conversions of intermediates in each biosynthetic pathway. Intermediates that exhibit multipotent behaviour have great potential for use as starting points in diversity-oriented synthesis. Inspired by the biosynthetic pathways that form complex metabolites from simple intermediates, we developed a semi-synthetic process that combines heterologous biosynthesis and artificial diversification. The heterologous biosynthesis of fungal polyketide intermediates led to the isolation of novel oligomers and provided evidence for ortho-quinonemethide equivalency in their isochromene form. The intrinsic reactivity of the isochromene polyketide enabled us to access various new chemical entities by modifying and remodelling the polyketide core and through coupling with indole molecules. We thus succeeded in generating exceptionally diverse pseudo-natural polyketides through this process and demonstrated an advanced method of using biosynthetic intermediates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Butler, M. S. The evolving role of natural products in drug discovery. Nature Rev. 4, 206–220 (2005).
Newman, D. J. & Cragg, G. M. Natural products as source of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75, 311–335 (2012).
Cragg, G. M. & Newman, D. J. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta 1830, 3670–3695 (2013).
Ortholand, J. Y. & Ganesan, A. Natural products and combinatorial chemistry: back to the future. Curr. Opin. Chem. Biol. 8, 271–280 (2004).
Rosén, J., Gottfries, J., Muresan, S., Backlund, A. & Oprea, T. I. Novel chemical space exploration via natural products. J. Med. Chem. 52, 1953–1962 (2009).
Eichner, S. et al. The interplay between mutasynthesis and semisynthesis: generation and evaluation of an ansamitocin library. Angew. Chem. Int. Ed. 51, 752–757 (2012).
Kirschning, A. & Hahn, F. Merging chemical synthesis and biosynthesis: a new chapter in the total synthesis of natural products and natural product libraries. Angew. Chem. Int. Ed. 51, 4012–4022 (2012).
Goss, R. J. M., Shankar, S. & Fayad, A. A. The generation of ‘unnatural’ products: synthetic biology meets synthetic chemistry. Nat. Prod. Rep. 29, 870–889 (2012).
Steinmetz, H. et al. Precursor-directed syntheses and biological evaluation of new elansolide derivatives. ChemBioChem 13, 1813–1817 (2012).
Yan, Y. et al. Multiplexing of combinatorial chemistry in antimycin biosynthesis: expression of molecular diversity and utility. Angew. Chem. Int. Ed. 52, 12308–12312 (2013).
Altmann, K. H., Gaugaz, F. Z. & Schiess, R. Diversity through semisynthesis: the chemistry and biological activity of semisynthetic epothilone derivatives. Mol. Divers. 15, 383–399 (2011).
Dupuis, S. N. et al. Synthetic diversification of natural products: semi-synthesis and evaluation of triazole jadomycins. Chem. Sci. 3, 1640–1644 (2012).
Ignatenko, V. A., Han, Y. & Tochtrop, G. P. Molecular library synthesis using complex substrates: expanding the framework of triterpenoids. J. Org. Chem. 78, 410–418 (2013).
Balthaser, B. R., Maloney, M. C., Beeler, A. B., Porco, J. A. & Snyder, J. K. Remodeling of the natural product fumagillol employing a reaction discovery approach. Nature Chem. 3, 969–973 (2011).
Huigens, R. W. et al. A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products. Nature Chem. 5, 195–202 (2013).
Keller, N. P., Turner, G. & Bennett, J. W. Fungal secondary metabolism from biochemistry to genomics. Nature Rev. Immunol. 3, 937–947 (2005).
Kharwar, R. N., Mishra, A., Gond, S. K., Stierle, A. & Stierle, D. Anticancer compounds derived from fungal endophytes: their importance and future challenges. Nat. Prod. Rep. 28, 1208–1228 (2011).
Brakhage, A. A. & Schroeckh, V. Fungal secondary metabolites—strategies to activate silent gene clusters. Fungal Genet. Biol. 48, 15–22 (2011).
Scherlach, K. & Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 7, 1753–1760 (2009).
Fisch, K. M. et al. Chemical induction of silent biosynthetic pathway transcription in Aspergillus niger. J. Ind. Microbiol. Biotechnol. 36, 1199–1213 (2009).
Khaldi, N. et al. SMURF: genomic mapping of fungal secondary metabolite clusters. Fungal Genet. Biol. 47, 736–741 (2010).
Tagami, K. et al. Reconstitution of biosynthetic machinery for indole–diterpene paxilline in Aspergillus oryzae. J. Am. Chem. Soc. 135, 1260–1263 (2013).
Chiang, Y. M. et al. An efficient system for heterologous expression of secondary metabolite genes in Aspergillus nidulans. J. Am. Chem. Soc. 135, 7720–7731 (2013).
Asai, T. et al. Structurally diverse chaetophenol productions induced by chemically mediated epigenetic manipulation of fungal gene expression. Org. Lett. 15, 3346–3349 (2013).
Asai, T., Taniguchi, T., Yamamoto, T., Monde, K. & Oshima, Y. Structures of spiroindicumides A and B, unprecedented carbon skeletal spirolactones, and determination of the absolute configuration by vibrational circular dichroism exciton approach. Org. Lett. 15, 4320–4323 (2013).
Asai, T., Yamamoto, T. & Oshima, Y. Aromatic polyketide production in Cordyceps indigotica, an entomopathogenic fungus, induced by exposure to a histone deacetylase inhibitor. Org. Lett. 14, 2006–2009 (2012).
Hashimoto, M. et al. Product identification of non-reducing polyketide synthases with C-terminus methyltransferase domain from Talaromyces stipitatus using Aspergillus oryzae heterologous expression. Bioorg. Med. Chem. Lett. 25, 1381–1384 (2015).
Liao, D., Li, H. & Lei, X. Efficient generation of ortho-quinone methide: application to the biomimetic syntheses of (±)-schefflone and tocopherol trimers. Org. Lett. 14, 18–21 (2012).
Steinhagen, H. & Corey, E. J. A convenient and versatile route to hydroquinolines by inter- and intramolecular aza-Diels–Alder pathways. Angew. Chem. Int. Ed. 38, 1928–1931 (1999).
O'Connor, S. E. & Maresh, J. J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 23, 532–547 (2006).
Xu, Z., Baunach, M., Ding, L. & Hertweck, C. Bacterial synthesis of diverse indole terpene alkaloids by an unparalleled cyclization sequence. Angew. Chem. Int. Ed. 51, 10293–10297 (2012).
Boettger, D. & Hertweck, C. Molecular diversity sculpted by fungal PKS–NRPS hybrids. ChemBioChem 14, 28–42 (2013).
Zi, W., Xie, W. & Ma, D. Total synthesis of akuammiline alkaloid (–)-vincorine via intramolecular oxidative coupling. J. Am. Chem. Soc. 134, 9126–9129 (2012).
Sandkovsky, U., Vargas, L. & Florescu, D. F. Adenovirus: current epidemiology and emerging approaches to prevention and treatment. Curr. Infect. Dis. Rep. 16, 416–423 (2014).
Naesens, L. et al. Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob. Agents Chemother. 49, 1010–1016 (2005).
Diaconu, I. et al. Human adenovirus replication in immunocompetent Syrian hamsters can be attenuated with chlorpromazine or cidofovir. J. Gene. Med. 12, 435–445 (2010).
Morita, H. et al. Synthesis of unnatural alkaloid scaffolds by exploiting plant polyketide synthase. Proc. Natl Acad. Sci. USA 108, 13504–13509 (2013).
Xu, Y. et al. Diversity-oriented combinatorial biosynthesis of benzenediol lactone scaffolds by subunit shuffling of fungal polyketide synthase. Proc. Natl Acad. Sci. USA 111, 12354–12359 (2014).
Hansen, D. A. et al. Biocatalytic synthesis of pikromycin, methymycin, neomethymycin, novamethymycin, and ketomethymycin. J. Am. Chem. Soc. 135, 11232–11238 (2013).
Newman, A. G., Vagstad, A. L., Storm, P. A. & Townsend, C. A. Systematic domain swaps of iterative, nonreducing polyketide synthases provide a mechanistic understanding and rationale for catalytic reprogramming. J. Am. Chem. Soc. 136, 7348–7362 (2014).
Harvey, C. J. B., Puglisi, J. D., Pande, V. S., Cane, D. E. & Khosla, C. Precursor directed biosynthesis of an orthogonally functional erythromycin analogue: selectivity in the ribosome macrolide binding pocket. J. Am. Chem. Soc. 134, 12259–12265 (2012).
Liu, T., Chiang, Y. M., Somoza, A. D., Oakley, B. R. & Wang, C. C. C. Engineering of an ‘unnatural’ natural product by swapping polyketide synthase domains in Aspergillus nidulans. J. Am. Chem. Soc. 133, 13314–13316 (2011).
Acknowledgements
This work was supported by JSPS KAKENHI (grant no. 25108702 to T.A. and 25293022 to Y.O.) from the Japan Society for the Promotion of Science (JSPS) and in part by the Platform for Drug Discovery, Informatics and Structural Life Science from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Contributions
T.A. conceived and designed the experiments, and carried out the experimental work, analysed the experimental results and wrote the manuscript. K.T., S.I. and N.S. performed the experimental work. K.G., M.H. and I.F. discussed the heterologous expression experiment. K.N. and E.K. examined the biological screening. Y.O. discussed all the results and provided oversight.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 9338 kb)
Rights and permissions
About this article
Cite this article
Asai, T., Tsukada, K., Ise, S. et al. Use of a biosynthetic intermediate to explore the chemical diversity of pseudo-natural fungal polyketides. Nature Chem 7, 737–743 (2015). https://doi.org/10.1038/nchem.2308
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2308
This article is cited by
-
Catalytic asymmetric oxa-Diels–Alder reaction of acroleins with simple alkenes
Nature Communications (2023)
-
Azaphilone alkaloids: prospective source of natural food pigments
Applied Microbiology and Biotechnology (2022)
-
Biomimetic approach to the catalytic enantioselective synthesis of tetracyclic isochroman
Nature Communications (2021)
-
Principle and design of pseudo-natural products
Nature Chemistry (2020)
-
Synthetic biology based construction of biological activity-related library of fungal decalin-containing diterpenoid pyrones
Nature Communications (2020)