Abstract
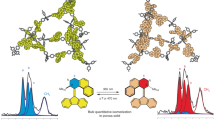
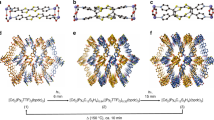
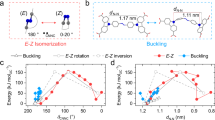
The development of solid materials that can be reversibly interconverted by light between forms with different physico-chemical properties is of great interest for separation, catalysis, optoelectronics, holography, mechanical actuation and solar energy conversion. Here, we describe a series of shape-persistent azobenzene tetramers that form porous molecular crystals in their E-configuration, the porosity of which can be tuned by changing the peripheral substituents on the molecule. Efficient E→Z photoisomerization of the azobenzene units takes place in the solid state and converts the crystals into a non-porous amorphous melt phase. Crystallinity and porosity are restored upon Z→E isomerization promoted by visible light irradiation or heating. We demonstrate that the photoisomerization enables reversible on/off switching of optical properties such as birefringence as well as the capture of CO2 from the gas phase. The linear design, structural versatility and synthetic accessibility make this new family of materials potentially interesting for technological applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kobatake, S., Takami, S., Muto, H., Ishikawa, T. & Irie, M. Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature 446, 778–781 (2007).
Szymanski, W., Beierle, J. M., Kistemaker, H. A. V., Velema, W. A. & Feringa, B. L. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem. Rev. 113, 6114–6178 (2013).
Bandara, H. M. D. & Burdette, S. C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 41, 1809–1825 (2012).
Bruder, F-K., Hagen, R., Rölle, T., Weiser, M-S. & Fäcke, T. From the surface to volume: concepts for the next generation of optical-holographic data-storage materials. Angew. Chem. Int. Ed. 50, 4552–4573 (2011).
Raimondo, C. et al. Optically switchable organic field-effect transistors based on photoresponsive gold nanoparticles blended with poly(3-hexylthiophene). Proc. Natl Acad. Sci. USA 109, 12375–12380 (2012).
Venkataramani, S. et al. Magnetic bistability of molecules in homogeneous solution at room temperature. Science 331, 445–448 (2011).
Ragazzon, G., Baroncini, M., Silvi, S., Venturi, M. & Credi, A. Light-powered autonomous and directional molecular motion based on a dissipative self-assembling system. Nature Nanotech. 10, 70–75 (2015).
Stoll, R. S. & Hecht, S. Artificial light-gated catalyst systems. Angew. Chem. Int. Ed. 49, 5054–5075 (2010).
Kausar, A., Nagano, H., Ogata, T., Nonaka, T. & Kurihara, S. Photocontrolled translational motion of a microscale solid object on azobenzene-doped liquid-crystalline films. Angew. Chem. Int. Ed. 48, 2144–2147 (2009).
Yanai, N. et al. Guest-to-host transmission of structural changes for stimuli-responsive adsorption property. J. Am. Chem. Soc. 134, 4501–4504 (2012).
Brown, J. W. et al. Photophysical pore control in an azobenzene-containing metal–organic framework. Chem. Sci. 4, 2858–2864 (2013).
Kucharski, T. J. et al. Templated assembly of photoswitches significantly increases the energy-storage capacity of solar thermal fuels. Nature Chem. 6, 441–447 (2014).
Velema, W. et al. Optical control of antibacterial activity. Nature Chem. 5, 924–928 (2013).
Naito, T., Horie, K. & Mita, I. Photochemistry in polymer solids. 11. The effects of the size of reaction groups and the mode of photoisomerization on photochromic reactions in polycarbonate film. Macromolecules 24, 2907–2911 (1991).
Evans, R. et al. The generic enhancement of photochromic dye switching speeds in a rigid polymer matrix. Nature Mater. 4, 249–253 (2005).
Tsuda, M. & Kuratani, K. Isomerization of cis-azobenzene in the solid phase. Bull. Chem. Soc. Jpn 37, 1284–1288 (1964).
Bushuyev, O. S., Tomberg, A., Friščić, T. & Barrett, C. J. Shaping crystals with light: crystal-to-crystal isomerization and photomechanical effect in fluorinated azobenzenes. J. Am. Chem. Soc. 135, 12556–12559 (2013).
Bléger, D., Yu, Z. & Hecht, S. Toward optomechanics: maximizing the photodeformation of individual molecules. Chem. Commun. 47, 12260–12266 (2011).
Lee, S. et al. Stimulus-responsive azobenzene supramolecules: fibers, gels, and hollow spheres. Langmuir 29, 5869–5877 (2013).
Yu, Y., Maeda, T., Mamiya, J-I. & Ikeda, T. Photomechanical effects of ferroelectric liquid-crystalline elastomers containing azobenzene chromophores. Angew. Chem. Int. Ed. 46, 881–883 (2007).
Norikane, Y., Hirai, Y. & Yoshida, M. Photoinduced isothermal phase transitions of liquid-crystalline macrocyclic azobenzenes. Chem. Commun. 47, 1770–1772 (2011).
Akiyama, H. & Yoshida, M. Photochemically reversible liquefaction and solidification of single compounds based on a sugar alcohol scaffold with multi azo-arms. Adv. Mater. 24, 2353–2356 (2012).
Uchida, E. et al. Control of the orientation and photoinduced phase transitions of macrocyclic azobenzene. Chem. Eur. J. 19, 17391–17397 (2013).
Hoshino, M. et al. Crystal melting by light: X-ray crystal structure analysis of an azo crystal showing photoinduced crystal-melt transition. J. Am. Chem. Soc. 136, 9158–9164 (2014).
Nakano, H., Seki, S. & Kageyama, H. Photoinduced vitrification near the surfaces of single crystals of azobenzene-based molecular materials with glass-forming ability. Phys. Chem. Chem. Phys. 12, 7772–7774 (2010).
Yan, X., Wang, F., Zheng, B. & Huang, F. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 41, 6042–6065 (2012).
Harada, A., Takashima, Y. & Nakahata, M. Supramolecular polymeric materials via cyclodextrin–guest interactions. Acc. Chem. Res. 47, 2128–2140 (2014).
Ichimura, K. Reversible photoisomerisability and particle size changes of mill-dispersed azobenzene crystals in water. Chem. Commun. 45, 1496–1498 (2009).
Brown, J. W. et al. Photophysical pore control in an azobenzene-containing metal–organic framework. Chem. Sci. 4, 2858 (2013).
Patel, H. et al. Unprecedented high-temperature CO2 selectivity in N2-phobic nanoporous covalent organic polymers. Nature Commun. 4, 1357 (2013).
Lyndon, R. et al. Dynamic photo-switching in metal–organic frameworks as a route to low-energy carbon dioxide capture and release. Angew. Chem. Int. Ed. 52, 3695–3698 (2013).
Park, J., Sun, L-B., Chen, Y-P., Perry, Z. & Zhou, H-C. Azobenzene-functionalized metal–organic polyhedra for the optically responsive capture and release of guest molecules. Angew. Chem. Int. Ed. 53, 5842–5846 (2014).
Patel, H. et al. Directing the structural features of N2-phobic nanoporous covalent organic polymers for CO2 capture and separation. Chem. Eur. J. 20, 772–780 (2014).
Koshima, H., Ojima, N. & Uchimoto, H. Mechanical motion of azobenzene crystals upon photoirradiation. J. Am. Chem. Soc. 131, 6890–6891 (2009).
Koshima, H. & Ojima, N. Photomechanical bending of 4-aminoazobenzene crystals. Dyes Pigm. 92, 798–801 (2012).
Bushuyev, O. S., Singleton, T. & Barrett, C. J. Fast, reversible, and general photomechanical motion in single crystals of various azo compounds using visible light. Adv. Mater. 25, 1796–1800 (2013).
Holst, J. R., Trewin, A. & Cooper, A. I. Porous organic molecules. Nature Chem. 2, 915–920 (2010).
McKeown, N. B. Nanoporous molecular crystals. J. Mater. Chem. 20, 10588–10597 (2010).
Sozzani, P., Bracco, S., Comotti, A., Ferretti, L. & Simonutti, R. Methane and carbon dioxide storage in a porous van der Waals crystal. Angew. Chem. Int. Ed. 44, 1816–1820 (2005).
Mastalerz, M. & Oppel, I. M. Rational construction of an extrinsic porous molecular crystal with an extraordinary high specific surface area. Angew. Chem. Int. Ed. 51, 5252–5255 (2012).
Zhou, H-C. & Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 43, 5415–5418 (2014).
Feng, X., Ding, X. & Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012).
Aujard, I. et al. Tetrahedral Onsager crosses for solubility improvement and crystallization bypass. J. Am. Chem. Soc. 123, 8177–8188 (2001).
Uyama, A. et al. Reversible photocontrol of surface wettability between hydrophilic and superhydrophobic surfaces on an asymmetric diarylethene solid surface. Langmuir 27, 6395–6400 (2011).
Palmer, B. et al. X-ray birefringence imaging. Science 344, 1013–1016 (2014).
Luo, F. et al. Photoswitching CO2 capture and release in a photochromic diarylethene metal–organic framework. Angew. Chem. Int. Ed. 53, 9298–9301 (2014).
Thallapally, P. K., Dalgarno, S. J. & Atwood, J. L. Frustrated organic solids display unexpected gas sorption. J. Am. Chem. Soc. 128, 15060–15061 (2006).
Jones, J. T. et al. On–off porosity switching in a molecular organic solid. Angew. Chem. Int. Ed. 50, 749–753 (2011).
Comotti, A. et al. Porous dipeptide crystals as selective CO2 adsorbents: experimental isotherms vs. grand canonical Monte Carlo simulations and MAS NMR spectroscopy. CrystEngComm. 15, 1503–1507 (2013).
Acknowledgements
This work was supported by Ministero dell'Istruzione, Università e Ricerca (PRIN 2010CX2TLM ‘InfoChem’ and FIRB 2010RBAP11C58Y ‘Nanosolar’), Ministero degli Affari Esteri e della Cooperazione Internazionale (DGSP n. 0075740), Fondazione Cariplo 2012-0921 and the University of Bologna. M.B. thanks R. Zamboni (ISOF-CNR Bologna) for support.
Author information
Authors and Affiliations
Contributions
M.B., A.C. and An.C. conceived the project. M.B. and T.M.H. synthesized and characterized the compounds. S.d'A. and F.G. performed crystal growth experiments and X-ray diffraction studies. S.S. and M.B. carried out the spectroscopic, photochemical and microscopy experiments. I.B., An.C. and P.S. performed gas adsorption experiments, solid-state NMR spectra and preparation and characterization of the polymorphs. G.B., P.C., M.V. and all other authors analysed the data. A.C. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 9484 kb)
Supplementary information
Crystallographic data for compound E4-1a. (CIF 193 kb)
Supplementary information
Crystallographic data for compound E4-1b Form I. (CIF 16 kb)
Supplementary information
Crystallographic data for compound E4-1b Form II. (CIF 218 kb)
Supplementary information
Crystallographic data for compound E4-1c. (CIF 232 kb)
Rights and permissions
About this article
Cite this article
Baroncini, M., d'Agostino, S., Bergamini, G. et al. Photoinduced reversible switching of porosity in molecular crystals based on star-shaped azobenzene tetramers. Nature Chem 7, 634–640 (2015). https://doi.org/10.1038/nchem.2304
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2304
This article is cited by
-
Recent progress in photoinduced transitions between the solid, glass, and liquid states based on molecular photoswitches
Polymer Journal (2024)
-
Solvophobicity-directed assembly of microporous molecular crystals
Communications Chemistry (2021)
-
Modulation of porosity in a solid material enabled by bulk photoisomerization of an overcrowded alkene
Nature Chemistry (2020)
-
Light-induced mechanical response in crosslinked liquid-crystalline polymers with photoswitchable glass transition temperatures
Nature Communications (2018)
-
Photochemical liquefaction and softening in molecular materials, polymers, and related compounds
Polymer Journal (2018)