Abstract
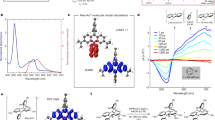
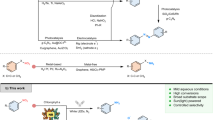
Artemisinin is an important antimalarial drug, but, at present, the environmental and economic costs of its semi-synthetic production are relatively high. Most of these costs lie in the final chemical steps, which follow a complex acid- and photo-catalysed route with oxygenation by both singlet and triplet oxygen. We demonstrate that applying the principles of green chemistry can lead to innovative strategies that avoid many of the problems in current photochemical processes. The first strategy combines the use of liquid CO2 as solvent and a dual-function solid acid/photocatalyst. The second strategy is an ambient-temperature reaction in aqueous mixtures of organic solvents, where the only inputs are dihydroartemisinic acid, O2 and light, and the output is pure, crystalline artemisinin. Everything else—solvents, photocatalyst and aqueous acid—can be recycled. Some aspects developed here through green chemistry are likely to have wider application in photochemistry and other reactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Anastas, P. T. & Warner, J. C. Green Chemistry: Theory and Practice (Oxford Univ. Press, 1998).
Tang, S. Y., Bourne, R. A., Smith, R. L. & Poliakoff, M. The 24 principles of green engineering and green chemistry: ‘IMPROVEMENTS PRODUCTIVELY’. Green Chem. 10, 268–269 (2008).
Dunn, P. J., Galvin, S. & Hettenbach, K. The development of an environmentally benign synthesis of sildenafil citrate (Viagra) and its assessment by green chemistry metrics. Green Chem. 6, 43–48 (2004).
Klayman, D. L. Qinghaosu (artemisinin): an antimalarial drug from China. Science 228, 1049–1055 (1985).
Paddon, C. J. & Keasling, J. D. Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nature Rev. Microbiol. 12, 355–367 (2014).
Westfall, P. J. et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc. Natl Acad. Sci. USA 109, E111–E118 (2012).
Bauer, A. & Bronstrup, M. Industrial natural product chemistry for drug discovery and development. Nat. Prod. Rep. 31, 35–60 (2014).
Paddon, C. J. et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496, 528–532 (2013).
Feth, M. P., Rossen, K. & Burgard, A. Pilot plant PAT approach for the diastereoselective diimide reduction of artemisinic acid. Org. Process Res. Dev. 17, 282–293 (2013).
Turconi, J. et al. Semisynthetic artemisinin, the chemical path to industrial production. Org. Process Res. Dev. 18, 417–422 (2014).
Roth, R. J. & Acton, N. A simple conversion of artemisinic acid into artemisinin. J. Nat. Prod. 52, 1183–1185 (1989).
Acton, N. & Roth, R. J. On the conversion of dihydroartemisinic acid into artemisinin. J. Org. Chem. 57, 3610–3614 (1992).
Kopetzki, D., Lévesque, F. & Seeberger, P. H. A continuous-flow process for the synthesis of artemisinin. Chem. Eur. J. 19, 5450–5456 (2013).
Lévesque, F. & Seeberger, P. H. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew. Chem. Int. Ed. 51, 1706–1709 (2012).
Sy, L-K. & Brown, G. D. The mechanism of the spontaneous autoxidation of dihydroartemisinic acid. Tetrahedron 58, 897–908 (2002).
Sy, L-K. & Brown, G. D. The role of the 12-carboxylic acid group in the spontaneous autoxidation of dihydroartemisinic acid. Tetrahedron 58, 909–923 (2002).
Singh, C., Chaudhary, S., Kanchan, R. & Puri, S. K. Conversion of antimalarial drug artemisinin to a new series of tricyclic 1,2,4-trioxanes. Org. Lett. 9, 4327–4329 (2007).
Worrall, D. R., Abdel-Shafi, A. A. & Wilkinson, F. Factors affecting the rate of decay of the first excited singlet state of molecular oxygen in supercritical fluid carbon dioxide. J. Phys. Chem. A 105, 1270–1276 (2001).
Leitner, W. & Jessop, P. G. (Eds) Handbook of Green Chemistry, Volume 4: Supercritical Solvents (Wiley-VCH, 2010).
Han, X. & Poliakoff, M. Continuous reactions in supercritical carbon dioxide: problems, solutions and possible ways forward. Chem. Soc. Rev. 41, 1428–1436 (2012).
Licence, P., Ke, J., Sokolova, M., Ross, S. K. & Poliakoff, M. Chemical reactions in supercritical carbon dioxide: from laboratory to commercial plant. Green Chem. 5, 99–104 (2003).
Bourne, R. A., Han, X., Poliakoff, M. & George, M. W. Cleaner continuous photo-oxidation using singlet oxygen in supercritical carbon dioxide. Angew. Chem. Int. Ed. 48, 5322–5325 (2009).
Han, X., Bourne, R. A., Poliakoff, M. & George, M. W. Immobilised photosensitisers for continuous flow reactions of singlet oxygen in supercritical carbon dioxide. Chem. Sci. 2, 1059–1067 (2011).
Han, X., Bourne, R. A., Poliakoff, M. & George, M. W. Strategies for cleaner oxidations using photochemically generated singlet oxygen in supercritical carbon dioxide. Green Chem. 11, 1787–1792 (2009).
Hall, J. F. B., Han, X., Poliakoff, M., Bourne, R. A. & George, M. W. Maximising the efficiency of continuous photo-oxidation with singlet oxygen in supercritical CO2 by use of fluorous biphasic catalysis. Chem. Commun. 48, 3073–3075 (2012).
Hall, J. F. B. et al. Synthesis of antimalarial trioxanes via continuous photo-oxidation with 1O2 in supercritical CO2 . Green Chem. 15, 177–180 (2013).
Griesbeck, A. G., El-Idreesy, T. T. & Bartoschek, A. Photooxygenation in polystyrene beads with covalently and non-covalently bound tetraarylporphyrin sensitizers. Adv. Synth. Catal. 346, 245–251 (2004).
Johnson Inbaraj, J., Vinodu, M. V., Gandhidasan, R., Murugesan, R. & Padmanabhan, M. Photosensitizing properties of ionic porphyrins immobilized on functionalized solid polystyrene support. J. Appl. Polym. Sci. 89, 3925–3930 (2003).
Ribeiro, S. M., Serra, A. C. & Rocha Gonsalves, A. M. d. A. Immobilised porphyrins in monoterpene photooxidations. J. Catal. 256, 331–337 (2008).
Ribeiro, S., Serra, A. C. & Rocha Gonsalves, A. M. d. A. Efficient solar photooxygenation with supported porphyrins as catalysts. ChemCatChem 5, 134–137 (2013).
Neuberger, A. & Scott, J. J. The basicities of the nitrogen atoms in the porphyrin nucleus; their dependence on some substituents of the tetrapyrrolic ring. Proc. R. Soc. Lond. Math. Phys. Sci. 213, 307–326 (1952).
Frederiksen, P. K. et al. Two-photon photosensitized production of singlet oxygen in water. J. Am. Chem. Soc. 127, 255–269 (2004).
Jensen, R. L., Arnbjerg, J. & Ogilby, P. R. Reaction of singlet oxygen with tryptophan in proteins: a pronounced effect of the local environment on the reaction rate. J. Am. Chem. Soc. 134, 9820–9826 (2012).
Yavorskyy, A. et al. Photooxygenations in a bubble column reactor. Green Chem. 14, 888–892 (2012).
Zepp, R. G., Wolfe, N. L., Baughman, G. L. & Hollis, R. C. Singlet oxygen in natural waters. Nature 267, 421–423 (1977).
Lindig, B. A., Rodgers, M. A. J. & Schaap, A. P. Determination of the lifetime of singlet oxygen in D2O using 9,10-anthracenedipropionic acid, a water-soluble probe. J. Am. Chem. Soc. 102, 5590–5593 (1980).
Jiménez-González, C., Constable, D. J. C. & Ponder, C. S. Evaluating the ‘greenness’ of chemical processes and products in the pharmaceutical industry—a green metrics primer. Chem. Soc. Rev. 41, 1485–1498 (2012).
Calvo-Flores, F. G. Sustainable chemistry metrics. ChemSusChem 2, 905–919 (2009).
Sheldon, R. A. The E factor: fifteen years on. Green Chem. 9, 1273–1283 (2007).
Sheldon, R. A. Consider the environmental quotient. Chemtech 24, 38–47 (1994).
Ciamician, G. The photochemistry of the future. Science 36, 385–394 (1912).
Freemantle, M. Ionic Liquids as Designer Solvents (Royal Society of Chemistry, 2010).
Acknowledgements
The authors acknowledge support from Sanofi, the University of Nottingham, the Bill and Melinda Gates Foundation (grant no. 1070294), and the Engineering and Physical Sciences Research Coucil (EPSRC; grant no. EP/L021889/1, ‘Continuous Chemical Manufacture with Light’). J.F.B.B. thanks the EPSRC for a studentship and M.W.G. thanks the Royal Society for a Wolfson Merit Award. The authors thank L. Hitchen for her contribution to batch studies in scCO2, M. Guyler, P. Fields, R. Wilson and D. Litchfield for technical support, G. Coxhill for MALDI analysis and W. Lewis for obtaining the crystal structure of 1 (shown in Fig. 3c). The authors thank D.B. Amabilino and P. Licence for helpful comments. Note that the production of semi-synthetic 1 at Sanofi is a not-for-profit venture, its development being supported in part by the Bill and Melinda Gates Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed directly to the project, and all were involved in the writing and revision of the paper. Z.A. led the organic chemistry experimental work and compound characterization. J.F.B.B. focused on the flow chemistry in scCO2. R.H. measured the singlet O2 kinetics, together with A.B., in A.B.'s laboratory in Durham. S.J.M. worked on the reactions in aqueous solvents. A.Bu. and K.R. provided technical advice regarding the overall process, as well as benchmarking against the current commercial process. M.P. and M.W.G. led the project and are responsible for the major part of writing this paper, but all authors discussed the results and commented on the various versions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4084 kb)
Supplementary information
Crystallographic data for compound 1. (CIF 1235 kb)
Supplementary information
Structure factors for compound 1. (CIF 163 kb)
Rights and permissions
About this article
Cite this article
Amara, Z., Bellamy, J., Horvath, R. et al. Applying green chemistry to the photochemical route to artemisinin. Nature Chem 7, 489–495 (2015). https://doi.org/10.1038/nchem.2261
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2261
This article is cited by
-
Kinetic effects in singlet oxygen mediated oxidations by immobilized photosensitizers on silica
Photochemical & Photobiological Sciences (2024)
-
Enhanced singlet oxygen production under nanoconfinement using silica nanocomposites towards improving the photooxygenation’s conversion
Journal of Nanoparticle Research (2022)
-
Imposed dynamic irradiation to intensify photocatalytic reactions
Journal of Flow Chemistry (2021)
-
Cobalt-catalyzed highly enantioselective hydrogenation of α,β-unsaturated carboxylic acids
Nature Communications (2020)
-
The Use of Molecular Oxygen for Liquid Phase Aerobic Oxidations in Continuous Flow
Topics in Current Chemistry (2019)