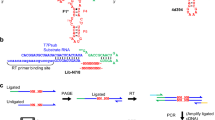
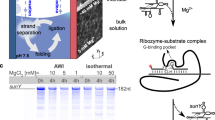
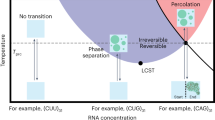
Abstract
The emergence of an RNA catalyst capable of self-replication is considered a key transition in the origin of life. However, how such replicase ribozymes emerged from the pools of short RNA oligomers arising from prebiotic chemistry and non-enzymatic replication is unclear. Here we show that RNA polymerase ribozymes can assemble from simple catalytic networks of RNA oligomers no longer than 30 nucleotides. The entropically disfavoured assembly reaction is driven by iterative freeze–thaw cycles, even in the absence of external activation chemistry. The steep temperature and concentration gradients of such cycles result in an RNA chaperone effect that enhances the otherwise only partially realized catalytic potential of the RNA oligomer pool by an order of magnitude. Our work outlines how cyclic physicochemical processes could have driven an expansion of RNA compositional and phenotypic complexity from simple oligomer pools.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gilbert, W. Origin of life: the RNA world. Nature 319, 618–618 (1986).
Powner, M. W., Gerland, B. & Sutherland, J. D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242 (2009).
Ferris, J. P., Hill, A. R. Jr, Liu, R. & Orgel, L. E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381, 59–61 (1996).
Huang, W. & Ferris, J. P. One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis. J. Am. Chem. Soc. 128, 8914–8919 (2006).
Monnard, P. A. & Szostak, J. W. Metal-ion catalyzed polymerization in the eutectic phase in water-ice: a possible approach to template-directed RNA polymerization. J. Inorg. Biochem. 102, 1104–1111, (2008).
Mansy, S. S. et al. Template-directed synthesis of a genetic polymer in a model protocell. Nature 454, 122–125 (2008).
Deck, C., Jauker, M. & Richert, C. Efficient enzyme-free copying of all four nucleobases templated by immobilized RNA. Nature Chem. 3, 603–608 (2011).
Adamala, K. & Szostak, J. W. Nonenzymatic template-directed RNA synthesis inside model protocells. Science 342, 1098–1100 (2013).
Morasch, M., Mast, C. B., Langer, J. K., Schilcher, P. & Braun, D. Dry polymerization of 3′,5′-cyclic GMP to long strands of RNA. ChemBioChem 15, 879–883 (2014).
Da Silva, L., Maurel, M. C. & Deamer, D. Salt-promoted synthesis of RNA-like molecules in simulated hydrothermal conditions. J. Mol. Evol. 80, 86–97 (2015).
Monnard, P. A., Kanavarioti, A. & Deamer, D. W. Eutectic phase polymerization of activated ribonucleotide mixtures yields quasi-equimolar incorporation of purine and pyrimidine nucleobases. J. Am. Chem. Soc. 125, 13734–13740 (2003).
Lincoln, T. A. & Joyce, G. F. Self-sustained replication of an RNA enzyme. Science 323, 1229–1232 (2009).
Vaidya, N. et al. Spontaneous network formation among cooperative RNA replicators. Nature 491, 72–77 (2012).
Doudna, J. A., Couture, S. & Szostak, J. W. A multisubunit ribozyme that is a catalyst of and template for complementary strand RNA synthesis. Science 251, 1605–1608 (1991).
Sczepanski, J. T. & Joyce, G. F. A cross-chiral RNA polymerase ribozyme. Nature 515, 440–442 (2014).
Johnston, W. K., Unrau, P. J., Lawrence, M. S., Glasner, M. E. & Bartel, D. P. RNA-catalyzed RNA polymerization: accurate and general RNA-templated primer extension. Science 292, 1319–1325 (2001).
Wochner, A., Attwater, J., Coulson, A. & Holliger, P. Ribozyme-catalyzed transcription of an active ribozyme. Science 332, 209–212 (2011).
Attwater, J., Wochner, A. & Holliger, P. In-ice evolution of RNA polymerase ribozyme activity. Nature Chem. 5, 1011–1018 (2013).
Szostak, J. W. The eightfold path to non-enzymatic RNA replication. J. Syst. Chem. 3, 2 (2012).
Engelhart, A. E., Powner, M. W. & Szostak, J. W. Functional RNAs exhibit tolerance for non-heritable 2′-5′ versus 3′-5′ backbone heterogeneity. Nature Chem. 5, 390–394 (2013).
Manrubia, S. C. & Briones, C. Modular evolution and increase of functional complexity in replicating RNA molecules. RNA 13, 97–107 (2007).
Briones, C., Stich, M. & Manrubia, S. C. The dawn of the RNA World: toward functional complexity through ligation of random RNA oligomers. RNA 15, 743–749 (2009).
Buzayan, J. M., Gerlach, W. L. & Bruening, G. Non-enzymatic cleavage and ligation of RNAs complementary to a plant virus satellite RNA. Nature 323, 349–353 (1986).
Kath-Schorr, S. et al. General acid-base catalysis mediated by nucleobases in the hairpin ribozyme. J. Am. Chem. Soc. 134, 16717–16724 (2012).
Butcher, S. E., Heckman, J. E. & Burke, J. M. Reconstitution of hairpin ribozyme activity following separation of functional domains. J. Biol. Chem. 270, 29648–29651 (1995).
Nesbitt, S. M., Erlacher, H. A. & Fedor, M. J. The internal equilibrium of the hairpin ribozyme: temperature, ion and pH effects. J. Mol. Biol. 286, 1009–1024 (1999).
Vlassov, A. V., Johnston, B. H., Landweber, L. F. & Kazakov, S. A. Ligation activity of fragmented ribozymes in frozen solution: implications for the RNA world. Nucleic Acids Res. 32, 2966–2974 (2004).
Kazakov, S. A., Balatskaya, S. V. & Johnston, B. H. Ligation of the hairpin ribozyme in cis induced by freezing and dehydration. RNA 12, 446–456 (2006).
Mutschler, H. & Holliger, P. Non-canonical 3′-5′ extension of RNA with prebiotically plausible ribonucleoside 2′,3′-cyclic phosphates. J. Am. Chem. Soc. 136, 5193–5196 (2014).
Attwater, J., Wochner, A., Pinheiro, V. B., Coulson, A. & Holliger, P. Ice as a protocellular medium for RNA replication. Nature Commun. 1, 76 (2010).
Zhuang, X. et al. Correlating structural dynamics and function in single ribozyme molecules. Science 296, 1473–1476 (2002).
Okumus, B., Wilson, T. J., Lilley, D. M. & Ha, T. Vesicle encapsulation studies reveal that single molecule ribozyme heterogeneities are intrinsic. Biophys. J. 87, 2798–2806 (2004).
Russell, R. RNA misfolding and the action of chaperones. Front. Biosci. 13, 1–20 (2008).
Bartel, D. P. & Szostak, J. W. Isolation of new ribozymes from a large pool of random sequences [see comment]. Science 261, 1411–1418 (1993).
Dropulic, B., Lin, N. H. & Jeang, K. T. A method to increase the cumulative cleavage efficiency of ribozymes: thermal cycling. Nucleic Acids Res. 21, 2273–2274 (1993).
Li, Y. & Breaker, R. R. Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2′-hydroxyl group. J. Am. Chem. Soc. 121, 5364–5372 (1999).
Eftink, M. R. & Biltonen, R. L. Energetics of ribonuclease A catalysis. 2. Nonenzymatic hydrolysis of cytidine cyclic 2′,3′-phosphate. Biochemistry 22, 5134–5140 (1983).
Murray, J. B., Seyhan, A. A., Walter, N. G., Burke, J. M. & Scott, W. G. The hammerhead, hairpin and VS ribozymes are catalytically proficient in monovalent cations alone. Chem. Biol. 5, 587–595 (1998).
Paudel, B. P. & Rueda, D. Molecular crowding accelerates ribozyme docking and catalysis. J. Am. Chem. Soc. 136, 16700–16703 (2014).
Hampel, K. J. & Burke, J. M. A conformational change in the ‘loop E-like’ motif of the hairpin ribozyme is coincidental with domain docking and is essential for catalysis. Biochemistry 40, 3723–3729 (2001).
Flores, R. et al. Rolling-circle replication of viroids, viroid-like satellite RNAs and hepatitis delta virus: variations on a theme. RNA Biol. 8, 200–206 (2011).
Riley, C. A. & Lehman, N. Generalized RNA-directed recombination of RNA. Chem. Biol. 10, 1233–1243 (2003).
Gabellieri, E. & Strambini, G. B. Perturbation of protein tertiary structure in frozen solutions revealed by 1-anilino-8-naphthalene sulfonate fluorescence. Biophys. J. 85, 3214–3220 (2003).
Strambini, G. B. & Gabellieri, E. Proteins in frozen solutions: evidence of ice-induced partial unfolding. Biophys. J. 70, 971–976 (1996).
Kolhe, P., Amend, E. & Singh, S. K. Impact of freezing on pH of buffered solutions and consequences for monoclonal antibody aggregation. Biotechnol. Prog. 26, 727–733 (2010).
Watanabe, H., Otsuka, T., Harada, M. & Okada, T. Imbalance between anion and cation distribution at ice interface with liquid phase in frozen electrolyte as evaluated by fluorometric measurements of pH. J. Phys. Chem. C 118, 15723–15731 (2014).
Miller, S. & Lazcano, A. The origin of life—did it occur at high temperatures? J. Mol. Evol. 41, 689–692 (1995).
Bada, J. L. How life began on Earth: a status report. Earth Planet. Sci. Lett. 226, 1–15 (2004).
Kreysing, M., Keil, L., Lanzmich, S. & Braun, D. Heat flux across an open pore enables the continuous replication and selection of oligonucleotides towards increasing length. Nature Chem. 7, 203–208 (2015).
Loffler, P. M., Groen, J., Dorr, M. & Monnard, P. A. Sliding over the blocks in enzyme-free RNA copying—one-pot primer extension in ice. PloS ONE 8, e75617 (2013).
Szostak, J. W. An optimal degree of physical and chemical heterogeneity for the origin of life? Phil. Trans. R. Soc. Lond. B 366, 2894–2901 (2011).
Meyer, A. J., Ellefson, J. W. & Ellington, A. D. Abiotic self-replication. Acc. Chem. Res. 45, 2097–2105 (2012).
Gollihar, J., Levy, M. & Ellington, A. D. Biochemistry. Many paths to the origin of life. Science 343, 259–260 (2014).
Acknowledgements
The authors thank J. Attwater for discussions and comments on the manuscript. This work was supported by a Federation of European Biochemical Societies (FEBS) long-term fellowship (H.M.) and by the Medical Research Council (A.W. and P.H., program no. U105178804).
Author information
Authors and Affiliations
Contributions
H.M., A.W. and P.H. conceived and designed the experiments. A.W. performed the in-ice invasion of the stem–loop structure (Supplementary Fig. 5). H.M. performed and analysed all the other experiments. All the authors discussed the results, and wrote and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 6101 kb)
Rights and permissions
About this article
Cite this article
Mutschler, H., Wochner, A. & Holliger, P. Freeze–thaw cycles as drivers of complex ribozyme assembly. Nature Chem 7, 502–508 (2015). https://doi.org/10.1038/nchem.2251
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2251
This article is cited by
-
Physical non-equilibria for prebiotic nucleic acid chemistry
Nature Reviews Physics (2023)
-
Ratcheting synthesis
Nature Reviews Chemistry (2023)
-
Periodic temperature changes drive the proliferation of self-replicating RNAs in vesicle populations
Nature Communications (2023)
-
Hydrophobic-cationic peptides modulate RNA polymerase ribozyme activity by accretion
Nature Communications (2022)
-
Frontiers in Prebiotic Chemistry and Early Earth Environments
Origins of Life and Evolution of Biospheres (2022)