Abstract
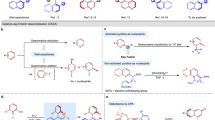
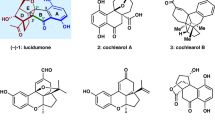
Chiral, dimeric natural products containing complex structures and interesting biological properties have inspired chemists and biologists for decades. A seven-step total synthesis of the axially chiral, dimeric tetrahydroxanthone natural product rugulotrosin A is described. The synthesis employs a one-pot Suzuki coupling/dimerization to generate the requisite 2,2′-biaryl linkage. Highly selective point-to-axial chirality transfer was achieved using palladium catalysis with achiral phosphine ligands. Single X-ray crystal diffraction data were obtained to confirm both the atropisomeric configuration and absolute stereochemistry of rugulotrosin A. Computational studies are described to rationalize the atropselectivity observed in the key dimerization step. Comparison of the crude fungal extract with synthetic rugulotrosin A and its atropisomer verified that nature generates a single atropisomer of the natural product.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Moss, G. P. Basic terminology of stereochemistry. Pure Appl. Chem. 68, 2193–2222 (1996).
Zask, A., Murphy, J. & Ellestad, G. A. Biological stereoselectivity of atropisomeric natural products and drugs. Chirality 25, 265–274 (2013).
Clayden, J., Moran, W. J., Edwards, P. J. & LaPlante, S. R. The challenge of atropisomerism in drug discovery. Angew. Chem. Int. Ed. 48, 6398–6401 (2009).
LaPlante, S. R., Edwards, P. J., Fader, L. D., Jakalian, A. & Hucke, O. Revealing atropisomer axial chirality in drug discovery. ChemMedChem 6, 505–513 (2011).
Bringmann, G. et al. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 44, 5387–5427 (2005).
Bringmann, G., Gulder, T., Gulder, T. A. M. & Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 111, 563–639 (2011).
Kozlowski, M. C., Morgan, B. J. & Linton, E. C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 38, 3193–3207 (2009).
Liau, B. B., Milgram, B. C. & Shair, M. D. Total syntheses of HMP-Y1, hibarimicinone, and HMP-P1. J. Am. Chem. Soc. 134, 16765–16772 (2012).
Masters, K-S. & Bräse, S. Xanthones from fungi, lichens, and bacteria: the natural products and their synthesis. Chem. Rev. 112, 3717–3776 (2012).
Wezeman, T., Masters, K-S. & Bräse, S. Double trouble—the art of synthesis of chiral dimeric natural products. Angew. Chem. Int. Ed. 53, 4524–4526 (2014).
Franck, B., Gottschalk, E. M., Ohnsorge, U. & Baumann, G. The structure of secalonic acids A and B. Angew. Chem. Int. Ed. Engl. 3, 441–442 (1964).
Steyn, P. S. The isolation, structure and absolute configuration of secalonic acid D, the toxic metabolite of Penicillium oxalicum. Tetrahedron 26, 51–57 (1970).
Stewart, M. et al. Rugulotrosins A and B: two new antibacterial metabolites from an Australian isolate of a Penicillium sp. J. Nat. Prod. 67, 728–730 (2004).
Kikuchi, H., Isobe, M., Kurata, S., Katou, Y. & Oshima, Y. New dimeric and monomeric chromanones, gonytolides D-G, isolated from the fungus Gonytrichum sp. Tetrahedron 68, 6218–6223 (2012).
Kikuchi, H. et al. Structures of the dimeric and monomeric chromanones, gonytolides A–C, isolated from the fungus Gonytrichum sp. and their promoting activities of innate immune responses. Org. Lett. 13, 4624–4627 (2011).
Nicolaou, K. C. & Li, A. Total syntheses and structural revision of α- and β-diversonolic esters and total syntheses of diversonol and blennolide C. Angew. Chem. Int. Ed. 47, 6579–6582 (2008).
Tietze, L. F. et al. Enantioselective total synthesis of (−)-diversonol. Chem. Eur. J. 19, 4876–4882 (2013).
Tietze, L. F., Ma, L., Reiner, J. R., Jackenkroll, S. & Heidemann, S. Enantioselective total synthesis of (−)-blennolide A. Chem. Eur. J. 19, 8610–8614 (2013).
Tietze, L. F., Jackenkroll, S., Hierold, J., Ma, L. & Waldecker, B. A domino approach to the enantioselective total syntheses of blennolide C and gonytolide C. Chem. Eur. J. 20, 8628–8635 (2014).
Nising, C. F., Ohnemüller, U. K. & Bräse, S. The total synthesis of the fungal metabolite diversonol. Angew. Chem. Int. Ed. 45, 307–309 (2006).
Meister, A. C. et al. Total synthesis of blennolide mycotoxins: design, synthetic routes and completion. Eur. J. Org. Chem. 4861–4875 (2014).
Qin, T., Johnson, R. P. & Porco, J. A. Jr Vinylogous addition of siloxyfurans to benzopyryliums: a concise approach to the tetrahydroxanthone natural products. J. Am. Chem. Soc. 133, 1714–1717 (2011).
Qin, T. & Porco, J. A. Jr Total syntheses of secalonic acids A and D. Angew. Chem. Int. Ed. 53, 3107–3110 (2014).
Wilson, J. M. & Cram, D. J. Chiral leaving groups induce asymmetry in syntheses of binaphthyls in nucleophilic aromatic substitution reactions. J. Am. Chem. Soc. 104, 881–884 (1982).
Evans, D. A. et al. Nonconventional stereochemical issues in the design of the synthesis of the vancomycin antibiotics: challenges imposed by axial and nonplanar chiral elements in the heptapeptide aglycons. Angew. Chem. Int. Ed. 37, 2704–2708 (1998).
Burns, N. Z., Krylova, Z. N., Hanroush, R. N. & Baran, P. S. Scalable total synthesis and biological evaluation of haouamine A and its atropisomer. J. Am. Chem. Soc. 131, 9172–9173 (2009).
Guo, F., Konkol, L. C. & Thomson, R. J. Enantioselective synthesis of biphenols from 1,4-diketones by traceless central-to-axial chirality exchange. J. Am. Chem. Soc. 133, 18–20 (2011).
Konkol, L. C., Guo, F., Sarjeant, A. A. & Thomson, R. J. Enantioselective total synthesis and studies into the configurational stability of bismurrayaquinone A. Angew. Chem. Int. Ed. 50, 9931–9934 (2011).
Park, Y. S. et al. Synthesis of (−)-viriditoxin: a 6,6′-binaphthopyran-2-one that targets the bacterial cell division protein FtsZ. Angew. Chem. Int. Ed. 50, 3730–3733 (2011).
Lipshutz, B. H. & Keith, L. M. A stereospecific, intermolecular biaryl-coupling approach to korupensamine A en route to the michellamines. Angew. Chem. Int. Ed. 38, 3530–3533 (1999).
Huang, S., Peterson, T. B. & Lipshutz, B. H. Total synthesis of (+)-korupensamine B via an atropselective intermolecular biaryl coupling. J. Am. Chem. Soc. 132, 14021–14023 (2010).
Coleman, R. S. & Grant, E. B. Atropdiastereoselective total synthesis of phleichrome and the protein kinase C inhibitor calphostin A. J. Am. Chem. Soc. 116, 8795–8796 (1994).
Broka, C. A. Total syntheses of phleichrome, calphostin A, and calphostin D. Unusual stereoselective and stereospecific reactions in the synthesis of perylenequinones. Tetrahedron Lett. 32, 859–862 (1991).
Birman, V. B. & Li, X. Homobenzotetramisole: an effective catalyst for kinetic resolution of aryl-cycloalkanols. Org. Lett. 10, 1115–1118 (2008).
Müller, C. E. & Schreiner, P. R. Organocatalytic enantioselective acyl transfer onto racemic as well as meso alcohols, amines, and thiols. Angew. Chem. Int. Ed. 50, 6012–6042 (2010).
Barder, T. E., Walker, S. D., Martinelli, J. R. & Buchwald, S. L. Catalysts for Suzuki–Miyaura coupling processes: scope and studies of the effect of ligand structure. J. Am. Chem. Soc. 127, 4685–4696 (2005).
Masamune, S., Choy, W., Petersen, J. S. & Sita, L. R. Double asymmetric synthesis and a new strategy for stereochemical control in organic synthesis. Angew. Chem. Int. Ed. Engl. 24, 1–30 (1985).
Tang, W. et al. A general and special catalyst for Suzuki–Miyaura coupling processes. Angew. Chem. Int. Ed. 49, 5879–5883 (2010).
Xu, G., Fu, W., Liu, G., Senanayake, C. H. & Tang, W. Efficient syntheses of korupensamines A, B and michellamine B by asymmetric Suzuki–Miyaura coupling reactions. J. Am. Chem. Soc. 136, 570–573 (2014).
Hamada, T., Chieffi, A., Ahman, J. & Buchwald, S. L. An improved catalyst for the asymmetric arylation of ketone enolates. J. Am. Chem. Soc. 124, 1261–1268 (2002).
Zhou, Y. et al. Enantioselective synthesis of axially chiral multifunctionalized biaryls via asymmetric Suzuki–Miyaura coupling. Org. Lett. 15, 5508–5511 (2013).
Zhou, Y. et al. Enantioselective synthesis of axially chiral biaryl monophosphine oxides via direct asymmetric Suzuki coupling and DFT investigations of the enantioselectivity. ACS Catal. 4, 1390–1397 (2014).
Bruno, N. C., Tudge, M. T. & Buchwald, S. L. Design and preparation of new palladium precatalysts for C–C and C–N cross-coupling reactions. Chem. Sci. 4, 916–920 (2013).
Little, S. & Trice, J. in Encyclopedia of Reagents for Organic Synthesis (Wiley, 2001); http://onlinelibrary.wiley.com/doi/10.1002/047084289X.rn01181/abstract
Molander, G. A., Trice, S. L. J., Kennedy, S. M., Dreher, S. D. & Tudge, M. T. Scope of the palladium-catalyzed aryl borylation utilizing bis-boronic acid. J. Am. Chem. Soc. 134, 11667–11673 (2012).
Gensch, T. et al. Snapshot of the palladium (II)-catalyzed oxidative biaryl bond formation by X-ray analysis of the intermediate diaryl palladium (II) complex. Chem. Eur. J. 18, 770–776 (2012).
Shen, X., Jones, G. O., Watson, D. A., Bhayana, B. & Buchwald, S. L. Enantioselective synthesis of axially chiral biaryls by the Pd-catalyzed Suzuki–Miyaura reaction: substrate scope and quantum mechanical investigation. J. Am. Chem. Soc. 132, 11278–11287 (2010).
Martin, R. & Buchwald, S. L. Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 41, 1461–1473 (2008).
Acknowledgements
Financial support from the National Institutes of Health (NIH, GM-099920) and Vertex Pharmaceuticals, Inc. (graduate fellowship to T.Q.) is gratefully acknowledged. The authors thank J. Bacon for crystal structure determination, B. Qu and C. Senanayake for providing both (R) and (S)-BI-DIME ligands and E. Lacey for supplying extracts of Penicillium nov. sp. (MST-F8741). Research at the BU-CMD was supported by the NIH (grant GM-067041). This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation (grant OCI-1053575).
Author information
Authors and Affiliations
Contributions
T.Q. and J.A.P. conceived of the project, designed and carried out the experiments, analysed the data and wrote most of the paper. S.L.S-J. and R.P.J. performed computational studies. Z.G.K. and R.J.C. performed natural extract comparisons and biological studies. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 7041 kb)
Supplementary information
Crystallographic data for compound (-)-19. (CIF 118 kb)
Supplementary information
Crystallographic data for compound (-)-22. (CIF 242 kb)
Rights and permissions
About this article
Cite this article
Qin, T., Skraba-Joiner, S., Khalil, Z. et al. Atropselective syntheses of (−) and (+) rugulotrosin A utilizing point-to-axial chirality transfer. Nature Chem 7, 234–240 (2015). https://doi.org/10.1038/nchem.2173
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2173
This article is cited by
-
Enantioselective construction of ortho-sulfur- or nitrogen-substituted axially chiral biaryls and asymmetric synthesis of isoplagiochin D
Nature Communications (2022)
-
Unusual dimeric tetrahydroxanthone derivatives from Aspergillus lentulus and the determination of their axial chiralities
Scientific Reports (2016)