Abstract
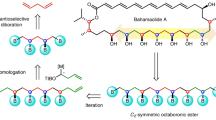
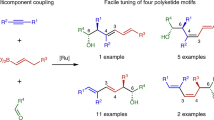
In planning organic syntheses, disconnections are most often made adjacent to functional groups, which assist in C–C bond formation. For molecules devoid of obvious functional groups this approach presents a problem, and so functionalities must be installed temporarily and then removed. Here we present a traceless strategy for organic synthesis that uses a boronic ester as such a group in a one-pot lithiation–borylation–protodeboronation sequence. To realize this strategy, we developed a methodology for the protodeboronation of alkyl pinacol boronic esters that involves the formation of a boronate complex with a nucleophile followed by oxidation with Mn(OAc)3 in the presence of the hydrogen-atom donor 4-tert-butylcatechol. Iterative lithiation–borylation–protodeboronation allows the coupling of smaller fragments to build-up long alkyl chains. We employed this strategy in the synthesis of hydroxyphthioceranic acid, a key component of the cell-wall lipid of the virulent Mycobacterium tuberculosis, in just 14 steps (longest linear sequence) with full stereocontrol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Damsté, J. S. S., Schouten, S., Hopmans, E. C., Duin, A. C. T. v. & Geenevasen, J. A. J. Crenarchaeol the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic Crenarchaeota. J. Lipid Res. 43, 1641–1651 (2002).
Stymiest, J., Dutheuil, G., Mahmood, A. & Aggarwal, V. Lithiated carbamates: chiral carbenoids for iterative homologation of boranes and boronic esters. Angew. Chem. Int. Ed. 46, 7491–7494 (2007).
Stymiest, J. L., Bagutski, V., French, R. M. & Aggarwal, V. K. Enantiodivergent conversion of chiral secondary alcohols into tertiary alcohols. Nature 456, 778–782 (2008).
Scott, H. K. & Aggarwal, V. K. Highly enantioselective synthesis of tertiary boronic esters and their stereospecific conversion to other functional groups and quaternary stereocentres. Chem. Eur. J. 17, 13124–13132 (2011).
Barluenga, J., Tomás-Gamasa, M., Aznar, F. & Valdés, C. Metal-free carbon–carbon bond-forming reductive coupling between boronic acids and tosylhydrazones. Nature Chem. 1, 494–499 (2009).
Myers, A. G. & Movassaghi, M. Highly efficient methodology for the reductive coupling of aldehyde tosylhydrazones with alkyllithium reagents. J. Am. Chem. Soc. 120, 8891–8892 (1998).
Shao, Z. & Zhang, H. N-Tosylhydrazones: versatile reagents for metal-catalyzed and metal-free cross-coupling reactions. Chem. Soc. Rev. 41, 560–572 (2012).
Mundal, D. A., Avetta C. T. Jr & Thomson, R. J. Triflimide-catalysed sigmatropic rearrangement of N-allylhydrazones as an example of a traceless bond construction. Nature Chem. 2, 294–297 (2010).
Nave, S., Sonawane, R., Elford, T. & Aggarwal, V. Protodeboronation of tertiary boronic esters: asymmetric synthesis of tertiary alkyl stereogenic centers. J. Am. Chem. Soc. 132, 17096–17098 (2010).
Roesner, S. & Aggarwal, V. K. Enantioselective synthesis of (R)-tolterodine using lithiation/borylation–protodeboronation methodology. Can. J. Chem. 90, 965–974 (2012).
Elford, T. G., Nave, S., Sonawane, R. P. & Aggarwal, V. K. Total synthesis of (+)-erogorgiaene using lithiation–borylation methodology, and stereoselective synthesis of each of its diastereoisomers. J. Am. Chem. Soc. 133, 16798–16801 (2011).
Roesner, S., Casatejada, J. M., Elford, T. G., Sonawane, R. P. & Aggarwal, V. K. Enantioselective syntheses of (+)-sertraline and (+)-indatraline using lithiation/borylation–protodeboronation methodology. Org. Lett. 13, 5740–5743 (2011).
Pozzi, D., Scanlan, E. M. & Renaud, P. A mild radical procedure for the reduction of B-alkylcatecholboranes to alkanes. J. Am. Chem. Soc 127, 14204–14205 (2005).
Villa, G., Povie, G. & Renaud, P. Radical chain reduction of alkylboron compounds with catechols. J. Am. Chem. Soc. 133, 5913–5920 (2011).
Brown, H. C. & Murray, K. J. Organoboranes for synthesis. 1: Protonolysis of trialkylboranes. A convenient non-catalytic conversion of alkenes into saturated compounds via hydroboration–protonolysis. Tetrahedron 42, 5497–5504 (1986).
Hesse, M. J., Butts, C. P., Willis, C. L. & Aggarwal, V. K. Diastereodivergent synthesis of trisubstituted alkenes through protodeboronation of allylic boronic esters: application to the synthesis of the Californian red scale beetle pheromone. Angew. Chem. Int. Ed. 51, 12444–12448 (2012).
Larouche-Gauthier, R., Elford, T. & Aggarwal, V. Ate complexes of secondary boronic esters as chiral organometallic-type nucleophiles for asymmetric synthesis. J. Am. Chem. Soc. 133, 16794–16797 (2011).
Sorin, G. et al. Oxidation of alkyl trifluoroborates: an opportunity for tin-free radical chemistry. Angew. Chem. Int. Ed. 49, 8721–8723 (2010).
Liu, K. E., Johnson, C. C., Newcomb, M. & Lippard, S. J. Radical clock substrate probes and kinetic isotope effect studies of the hydroxylation of hydrocarbons by methane monooxygenase. J. Am. Chem. Soc. 115, 939–947 (1993).
World Health Organization. WHO Global Tuberculosis Report – Executive Summary (2013). www.who.int/tb/publications/factsheet_global.pdf
World Health Organization. WHO Fact Sheets on Tuberculosis (2009). www.who.int/tb/publications/2009/tbfactsheet_2009update_one_page.pdf
Zhang, L., Goren, M. B., Holzer, T. J. & Andersen, B. R. Effect of Mycobacterium tuberculosis-derived sulfolipid I on human phagocytic cells. Infect. Immun. 56, 2876–2883 (1988).
Glickman, M. S. & Jacobs, W. R. Jr. Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline. Cell 104, 477–485 (2001).
Converse, S. E. et al. MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence. Proc. Natl Acad. Sci. USA 100, 6121–6126 (2003).
Goren, M. B., Brokl, O., Das, B. C. & Lederer, E. Sulfolipid I of Mycobacterium tuberculosis, strain H37RV. Nature of the acyl substituents. Biochemistry 10, 72–81 (1971).
Goren, M. B., Brokl, O., Roller, P., Fales, H. M. & Das, B. C. Sulfatides of Mycobacterium tuberculosis: the structure of the principal sulfatide (SL-I). Biochemistry 15, 2728–2735 (1976).
Young, D. & Dye, C. The development and impact of tuberculosis vaccines. Cell 124, 683–687 (2006).
Geerdink, D. et al. Total synthesis, stereochemical elucidation and biological evaluation of Ac2SGL; a 1,3-methyl branched sulfoglycolipid from Mycobacterium tuberculosis. Chem. Sci. 4, 709–716 (2013).
López, F., Minnaard, A. J. & Feringa, B. L. Catalytic enantioselective conjugate addition with Grignard reagents. Acc. Chem. Res. 40, 179–188 (2007).
Bjorn, T. H., Feringa, B. L. & Minnaard, A. J. Catalytic asymmetric synthesis of phthioceranic acid, a heptamethyl-branched acid from Mycobacterium tuberculosis. Org. Lett. 9, 3013–3015 (2007).
Geerdink, D. & Minnaard, A. J. Total synthesis of sulfolipid-1. Chem. Commun. 50, 2286–2288 (2014).
Pischl, M. C., Weise, C. F., Müller, M-A., Pfaltz, A. & Schneider, C. A convergent and stereoselective synthesis of the glycolipid components phthioceranic acid and hydroxyphthioceranic acid. Angew. Chem. Int. Ed. 52, 8968–8972 (2013).
Wang, Y. F., Chen, C. S., Girdaukas, G. & Sih, C. J. Bifunctional chiral synthons via biochemical methods. III. Optical purity enhancement in enzymic asymmetric catalysis. J. Am. Chem. Soc. 106, 3695–3696 (1984).
Schmidt, Y. et al. Enantioselective total synthesis of the unnatural and the natural stereoisomers of vittatalactone. J. Org. Chem. 75, 4424–4433 (2010).
Tsuji, K., Terao, Y. & Achiwa, K. Lipase-catalyzed asymmetric synthesis of chiral 1,3-propanediols and its application to the preparation of optically pure building block for renin inhibitors. Tetrahedron Lett. 30, 6189–6192 (1989).
Roesner, S. et al. Stereospecific conversion of alcohols into pinacol boronic esters using lithiation–borylation methodology with pinacolborane. Chem. Commun. 50, 4053–4055 (2014).
Dearden, M. J., Firkin, C. R., Hermet, J-P. R. & O'Brien, P. A readily-accessible (+)-sparteine surrogate. J. Am. Chem. Soc. 124, 11870–11871 (2002).
Dixon, A. J., Mcgrath, M. J. & O'Brien, P. Synthesis of (+)-(1R,2S,9S)-11-methyl-7,11-diazatricyclo[7.3.1.0]tridecane, a (+)-sparteine surrogate. Org. Synth. 83, 141–154 (2006).
Testa, M. L. et al. Oxidation of amino diols mediated by homogeneous and heterogeneous TEMPO. Adv. Synth. Catal. 346, 655–660 (2004).
Inokuchi, T., Matsumoto, S., Nishiyama, T. & Torii, S. A selective and efficient method for alcohol oxidations mediated by N-oxoammonium salts in combination with sodium bromite. J. Org. Chem. 55, 462–466 (1990).
Zhao, M. M., Li, J., Mano, E., Song, Z. J. & Tschaen, D. M. Oxidation of primary alcohols to carboxylic acids with sodium chlorite catalyzed by TEMPO and bleach: 4-methoxyphenylacetic acid. Org. Synth. 81, 195–203 (2005).
Acknowledgements
We thank the Engineering and Physical Science Research Council (EP/I038071/1) and the European Research Council (FP7/2007-2013, ERC grant no. 246785) for financial support. R.R. thanks the Marie Curie Fellowship program (EC FP7, No 329578). We thank A. Scott for technical assistance.
Author information
Authors and Affiliations
Contributions
V.K.A. conceived the project and wrote the manuscript with R.R. R.R planned and carried out the experiments. R.R. and V.K.A. discussed the experiments and results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 14532 kb)
Rights and permissions
About this article
Cite this article
Rasappan, R., Aggarwal, V. Synthesis of hydroxyphthioceranic acid using a traceless lithiation–borylation–protodeboronation strategy. Nature Chem 6, 810–814 (2014). https://doi.org/10.1038/nchem.2010
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2010
This article is cited by
-
Lithiation–borylation methodology in the total synthesis of natural products
Nature Synthesis (2022)
-
Iterative reactions of transient boronic acids enable sequential C–C bond formation
Nature Chemistry (2016)