Abstract
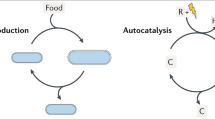
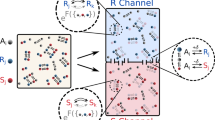
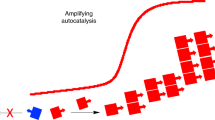
The route by which the complex and specific molecules of life arose from the 'prebiotic soup' remains an unsolved problem. Evolution provides a large part of the answer, but this requires molecules that can carry information (that is, exist in many variants) and can replicate themselves. The process is commonplace in living organisms, but not so easy to achieve with simple chemical systems. It is especially difficult to contemplate in the chemical chaos of the prebiotic world. Although popular in many quarters, the notion that RNA was the first self-replicator carries many difficulties. Here, we present an alternative view, suggesting that there may be undiscovered self-replication mechanisms possible in much simpler systems. In particular, we highlight the possibility of information coding through stereochemical configurations of substituents in organic polymers. We also show that this coding system leads naturally to enantiopurity, solving the apparent problem of biological homochirality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zimmer, C. How and where did life on Earth arise? Science 309, 89–89 (2005).
Lynn, D., Burrows, C., Goodwin, J. & Mehta, A. Origins of chemical evolution. Acc. Chem. Res. 45, 2023–2024 (2012).
McCollom, T. M. & Seewald, J. S. Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem. Rev. 107, 382–401 (2007).
Bada, J. L. New insights into prebiotic chemistry from Stanley Miller's spark discharge experiments. Chem. Soc. Rev. 42, 2186–2196 (2013).
Herd, C. D. K. et al. Origin and evolution of prebiotic organic matter as inferred from the Tagish Lake meteorite. Science 332, 1304–1307 (2011).
Benner, S. A., Ricardo, A. & Carrigan, M. A. Is there a common chemical model for life in the universe? Curr. Opin. Chem. Biol. 8, 672–689 (2004).
Atkins, J. F., Gesteland, R. F. & Cech, T. R. (eds) RNA Worlds: From Life's Origins to Diversity in Gene Regulation (Cold Spring Harbour Laboratory Press, 2011).
Ban, N., Nissen, P., Hansen, J., Moore, P. B. & Steitz, T. A. The complete atomic structure of the large ribosomal subunit at 2.4 angstrom resolution. Science 289, 905–920 (2000).
Wimberly, B. T. et al. Structure of the 30S ribosomal subunit. Nature 407, 327–339 (2000).
de Duve, C. The onset of selection. Nature 433, 581–582 (2005).
Joyce, G. F. & Orgel, L. E. in The RNA World (eds Gesteland, R. F., Cech, T. R. & Atkins, J. F.) 23–56 (Cold Spring Harbour Laboratory Press, 2006).
Orgel, L. E. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 39, 99–123 (2004).
Cairns-Smith, A. G. Genetic Takeover and the Mineral Origin of Life (Cambridge Univ. Press, 1982).
Cairns-Smith, A. G. Chemistry and the missing era of evolution. Chem. Eur. J. 14, 3830–3839 (2008).
Benner, S. A., Kim, H. J. & Carrigan, M. A. Asphalt, water, and the prebiotic synthesis of ribose, ribonucleosides, and RNA. Acc. Chem. Res. 45, 2025–2034 (2012).
Ricardo, A., Carrigan, M. A., Olcott, A. N. & Benner, S. A. Borate minerals stabilize ribose. Science 303, 196–196 (2004).
Lambert, J. B., Gurusamy-Thangavelu, S. A. & Ma, K. B. A. The silicate-mediated formose reaction: bottom-up synthesis of sugar silicates. Science 327, 984–986 (2010).
Ritson, D. & Sutherland, J. D. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nature Chem. 4, 895–899 (2012).
Powner, M. W., Gerland, B. & Sutherland, J. D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242 (2009).
Hein, J. E., Tse, E. & Blackmond, D. G. A route to enantiopure RNA precursors from nearly racemic starting materials. Nature Chem. 3, 704–706 (2011).
Bean, H. D. et al. Formation of a beta-pyrimidine nucleoside by a free pyrimidine base and ribose in a plausible prebiotic reaction. J. Am. Chem. Soc. 129, 9556–9557 (2007).
Huang, W. H. & Ferris, J. P. One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis. J. Am. Chem. Soc. 128, 8914–8919 (2006).
Deck, C., Jauker, M. & Richert, C. Efficient enzyme-free copying of all four nucleobases templated by immobilized RNA. Nature Chem. 3, 603–608 (2011).
Bowler, F. R. et al. Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation. Nature Chem. 5, 383–389 (2013).
Engelhart, A. E., Powner, M. W. & Szostak, J. W. Functional RNAs exhibit tolerance for non-heritable 2′-5′ versus 3′-5′ backbone heterogeneity. Nature Chem. 5, 390–394 (2013).
Joyce, G. F. et al. Chiral selection in poly(C)-directed synthesis of oligo(G). Nature 310, 602–604 (1984).
Joyce, G. F. Forty years of in vitro evolution. Angew. Chem. Int. Ed. 46, 6420–6436 (2007).
Robertson, M. P. & Scott, W. G. The structural basis of ribozyme-catalyzed RNA assembly. Science 315, 1549–1553 (2007).
Wochner, A., Attwater, J., Coulson, A. & Holliger, P. Ribozyme-catalyzed transcription of an active ribozyme. Science 332, 209–212 (2011).
Eschenmoser, A. Chemical etiology of nucleic acid structure. Science 284, 2118–2124 (1999).
Eschenmoser, A. Etiology of potentially primordial biomolecular structures: from vitamin B12 to the nucleic acids and an inquiry into the chemistry of life's origin: a retrospective. Angew. Chem. Int. Ed. 50, 12412–12472 (2011).
Bohler, C., Nielsen, P. E. & Orgel, L. E. Template switching between PNA and RNA oligonucleotides. Nature 376, 578–581 (1995).
Meggers, E. & Zhang, L. Synthesis and properties of the simplified nucleic acid glycol nucleic acid. Acc. Chem. Res. 43, 1092–1102 (2010).
Pinheiro, V. B. et al. Synthetic genetic polymers capable of heredity and evolution. Science 336, 341–344 (2012).
Hirao, I., Kimoto, M. & Yamashige, R. Natural versus artificial creation of base pairs in DNA: origin of nucleobases from the perspectives of unnatural base pair studies. Acc. Chem. Res. 45, 2055–2065 (2012).
Kostler, S. Polyaldehydes: homopolymers, block copolymers and promising applications. Polym. Int. 61, 1221–1227 (2012).
Danger, G., Plasson, R. & Pascal, R. Pathways for the formation and evolution of peptides in prebiotic environments. Chem. Soc. Rev. 41, 5416–5429 (2012).
Nampoothiri, K. M., Nair, N. R. & John, R. P. An overview of the recent developments in polylactide (PLA) research. Bioresource Technol. 101, 8493–8501 (2010).
Weber, A. L. The triose model: glyceraldehyde as a source of energy and monomers for prebiotic condensation-reactions. Origins Life Evol. B 17, 107–119 (1987).
Orgel, L. E. Unnatural selection in chemicals systems. Acc. Chem. Res. 28, 109–118 (1995).
Feringa, B. L. & van Delden, R. A. Absolute asymmetric synthesis: the origin, control, and amplification of chirality. Angew. Chem. Int. Ed. 38, 3419–3438 (1999).
Quack, M. How important is parity violation for molecular and biomolecular chirality? Angew. Chem. Int. Ed. 41, 4618–4630 (2002).
Pizzarello, S. The chemistry of life's origin: a carbonaceous meteorite perspective. Acc. Chem. Res. 39, 231–237 (2006).
Evans, A. C., Meinert, C., Giri, C., Goesmann, F. & Meierhenrich, U. J. Chirality, photochemistry and the detection of amino acids in interstellar ice analogues and comets. Chem. Soc. Rev. 41, 5447–5458 (2012).
Kondepudi, D. K. & Asakura, K. Chiral autocatalysis, spontaneous symmetry breaking, and stochastic behavior. Acc. Chem. Res. 34, 946–954 (2001).
Nanita, S. C. & Cooks, R. G. Serine octamers: cluster formation, reactions, and implications for biomolecule homochirality. Angew. Chem. Int. Ed. 45, 554–569 (2006).
Blackmond, D. G. Asymmetric autocatalysis and its implications for the origin of homochirality. Proc. Natl Acad. Sci. USA 101, 5732–5736 (2004).
Hein, J. E. & Blackmond, D. G. On the origin of single chirality of amino acids and sugars in biogenesis. Acc. Chem. Res. 45, 2045–2054 (2012).
Bolli, M., Micura, R. & Eschenmoser, A. Pyranosyl-RNA: chiroselective self-assembly of base sequences by ligative oligomerization of tetranucleotide-2′, 3′- cyclophosphates (with a commentary concerning the origin of biomolecular homochirality). Chem. Biol. 4, 309–320 (1997).
Siegel, J. S. Homochiral imperative of molecular evolution. Chirality 10, 24–27 (1998).
Schomaker, E. & Challa, G. Complexation of stereoregular poly(methyl methacrylates). 14. The basic structure of the stereocomplex of isotactic and syndiotactic poly(methyl methacrylate). Macromolecules 22, 3337–3341 (1989).
Serizawa, T. et al. Stepwise stereocomplex assembly of stereoregular poly(methyl methacrylate)s on a substrate. J. Am. Chem. Soc. 122, 1891–1899 (2000).
Serizawa, T., Hamada, K. & Akashi, M. Polymerization within a molecular-scale stereoregular template. Nature 429, 52–55 (2004).
Weissbuch, I., Illos, R. A., Bolbach, G. & Lahav, M. Racemic beta-sheets as templates of relevance to the origin of homochirality of peptides: lessons from crystal chemistry. Acc. Chem. Res. 42, 1128–1140 (2009).
Saghatelian, A., Yokobayashi, Y., Soltani, K. & Ghadiri, M. R. A chiroselective peptide replicator. Nature 409, 797–801 (2001).
Acknowledgements
We are grateful to the Leverhulme Trust for financial support of our work in this area (grant number F/00 182/CM).
Author information
Authors and Affiliations
Contributions
This article was written by A.P.D. with assistance, comments and criticism from A.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Brewer, A., Davis, A. Chiral encoding may provide a simple solution to the origin of life. Nature Chem 6, 569–574 (2014). https://doi.org/10.1038/nchem.1981
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1981
This article is cited by
-
Reprogramming biocatalytic futile cycles through computational engineering of stereochemical promiscuity to create an amine racemase
Nature Communications (2024)
-
Chiral recognition of neutral guests by chiral naphthotubes with a bis-thiourea endo-functionalized cavity
Nature Communications (2023)
-
Hyperpositive nonlinear effects in asymmetric catalysis
Nature Catalysis (2020)
-
Asymmetric assembly of aldose carbohydrates from formaldehyde and glycolaldehyde by tandem biocatalytic aldol reactions
Nature Chemistry (2015)