Abstract
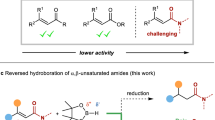
The cross-coupling of boronic acids and related derivatives with sp2 electrophiles (the Suzuki–Miyaura reaction) is one of the most powerful C–C bond formation reactions in synthesis, with applications that span pharmaceuticals, agrochemicals and high-tech materials. Despite the breadth of its utility, the scope of this Nobel prize-winning reaction is rather limited when applied to aliphatic boronic esters. Primary organoboron reagents work well, but secondary and tertiary boronic esters do not (apart from a few specific and isolated examples). Through an alternative strategy, which does not involve using transition metals, we have discovered that enantioenriched secondary and tertiary boronic esters can be coupled to electron-rich aromatics with essentially complete enantiospecificity. As the enantioenriched boronic esters are easily accessible, this reaction should find considerable application, particularly in the pharmaceutical industry where there is growing awareness of the importance of, and greater clinical success in, creating biomolecules with three-dimensional architectures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Suzuki, A. Cross-coupling reactions of organoboranes: an easy way to construct C–C bonds (Nobel Lecture). Angew. Chem. Int. Ed. 50, 6722–6737 (2011).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Molander, G. A. & Wisniewski, S. R. Stereospecific cross-coupling of secondary organotrifluoroborates: potassium 1-(benzyloxy)alkyltrifluoroborates. J. Am. Chem. Soc. 134, 16856–16868 (2012).
Awano, T., Ohmura, T. & Suginome, M. Inversion or retention? Effects of acidic additives on the stereochemical course in enantiospecific Suzuki–Miyaura coupling of α-(acetylamino)benzylboronic esters. J. Am. Chem. Soc. 133, 20738–20741 (2011).
Ohmura, T., Awano, T. & Suginome, M. Stereospecific Suzuki−Miyaura coupling of chiral α-(acylamino)benzylboronic esters with inversion of configuration. J. Am. Chem. Soc. 132, 13191–13193 (2010).
Sandrock, D. L., Jean-Gérard, L., Chen, C-Y., Dreher, S. D. & Molander, G. A. Stereospecific cross-coupling of secondary alkyl β-trifluoroboratoamides. J. Am. Chem. Soc. 132, 17108–17110 (2010).
Imao, D., Glasspoole, B. W., Laberge, V. S. & Crudden, C. M. Cross coupling reactions of chiral secondary organoboronic esters with retention of configuration. J. Am. Chem. Soc. 131, 5024–5025 (2009).
Lee, J. C. H., McDonald, R. & Hall, D. G. Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds. Nature Chem. 3, 894–899 (2011).
Zhou, S-M., Deng, M-Z., Xia, L-J. & Tang, M-H. Efficient Suzuki-type cross-coupling of enantiomerically pure cyclopropylboronic acids. Angew. Chem. Int. Ed. 37, 2845–2847 (1998).
Partridge, B. M., Chausset-Boissarie, L., Burns, M., Pulis, A. P. & Aggarwal, V. K. Enantioselective synthesis and cross-coupling of tertiary propargylic boronic esters using lithiation–borylation of propargylic carbamates. Angew. Chem. Int. Ed. 51, 11795–11799 (2012).
Chausset-Boissarie, L. et al. Enantiospecific, regioselective cross-coupling reactions of secondary allylic boronic esters. Chem. Eur. J. 19, 17698–17701 (2013).
Lovering, F., Bikker, J. & Humblet, C. Escape from Flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Dreher, S. D., Dormer, P. G., Sandrock, D. L. & Molander, G. A. Efficient cross-coupling of secondary alkyltrifluoroborates with aryl chlorides—reaction discovery using parallel microscale experimentation. J. Am. Chem. Soc. 130, 9257–9259 (2008).
Kataoka, N., Shelby, Q., Stambuli, J. P. & Hartwig, J. F. Air stable, sterically hindered ferrocenyl dialkylphosphines for palladium-catalyzed C–C, C–N, and C–O bond-forming cross-couplings. J. Org. Chem. 67, 5553–5566 (2002).
Swift, E. C. & Jarvo, E. R. Asymmetric transition metal-catalyzed cross-coupling reactions for the construction of tertiary stereocenters. Tetrahedron 69, 5799–5817 (2013).
Saito, B. & Fu, G. C. Enantioselective alkyl–alkyl Suzuki cross-couplings of unactivated homobenzylic halides. J. Am. Chem. Soc. 130, 6694–6695 (2008).
Zhou, J. & Fu, G. C. Cross-couplings of unactivated secondary alkyl halides: room-temperature nickel-catalyzed Negishi reactions of alkyl bromides and iodides. J. Am. Chem. Soc. 125, 14726–14727 (2003).
Son, S. & Fu, G. C. Nickel-catalyzed asymmetric Negishi cross-couplings of secondary allylic chlorides with alkylzincs. J. Am. Chem. Soc. 130, 2756–2757 (2008).
Cordier, C-J., Lundgren, R. J. & Fu, G. C. Enantioconvergent cross-couplings of racemic alkylmetal reagents with unactivated secondary alkyl electrophiles: catalytic asymmetric Negishi α-alkylations of N-Boc-pyrrolidine. J. Am. Chem. Soc. 135, 10946–10949 (2013).
Li, L., Wang, C-Y., Huang, R. & Biscoe, M. R. Stereoretentive Pd-catalysed Stille cross-coupling reactions of secondary alkyl azastannatranes and aryl halides. Nature Chem. 5, 607–612 (2013).
Kalkofen, R. & Hoppe, D. First example of an enantiospecific sp3–sp2 Stille coupling of a chiral allylstannane with aryl halides. Synlett 12, 1959–1961 (2006).
Falk, J. R., Patel, P. K. & Bandyopadhyay, A. Stereospecific cross-coupling of alpha-(thiocarbamoyl)organostannanes with alkenyl, aryl, and heteroaryl iodides. J. Am. Chem. Soc. 129, 790–793 (2007).
Yonova, I. M. et al. Stereospecific nickel-catalyzed cross-coupling reactions of alkyl Grignard reagents and identification of selective anti-breast-cancer agents. Angew. Chem. Int. Ed. 129, 2454–2459 (2014).
Zhou, Q., Srinivas, H. D., Dasgupta, S. & Watson, M. P. Nickel-catalyzed cross-couplings of benzylic pivalates with arylboroxines: stereospecific formation of diarylalkanes and triarylmethanes. J. Am. Chem. Soc. 135, 3307–3310 (2013).
Levy, A. B. Aromatic substitution via organoboranes. Regiospecific formation of 2-alkylindoles. J. Org. Chem. 43, 4684–4685 (1978).
Ishikura, M. & Kato, M. A synthetic use of the intramolecular alkyl migration process in indolylborates for intramolecular cyclization: a novel construction of carbazole derivatives. Tetrahedron 58, 9827–9838 (2002).
Akimoto, I. & Suzuki, A. Synthesis of 2-alkylfurans via the reaction of iodine with the ate-complexes obtained from 2-furyllithium and trialkylboranes. Synthesis 146–148 (1979).
Pelter, A., Williamson, H. & Davies, G. M. Aryl coupling through borate complexes with ethanolamine. Tetrahedron Lett. 25, 453–456 (1984).
Marinelli, E. R. & Levy, A. B. Aromatic substitution via organoboranes 2. Regiospecific alkylation of the furan and pyrrole nucleus. Tetrahedron Lett. 20, 2313–2316 (1979).
Ishikura, M., Kato, M. & Ohnuki, N. A novel C–C bond formation reaction with 1-methoxymethylindolylborate. Chem. Commun. 220–221 (2002).
Kagan, J. & Arora, S. K. The synthesis of alpha-thiophene oligomers via organoboranes. Tetrahedron Lett. 24, 4043–4046 (1983).
Davies, G. M., Davies, P. S., Paget, W. E. & Wardleworth, J. M. Borinic acids: novel intermediates in regiospecific synthesis of biaryls. Tetrahedron Lett. 17, 795–798 (1976).
Labadie, S. S. & Teng, E. Indol-2-yltributylstannane: a versatile reagent for 2-substituted indoles. J. Org. Chem. 59, 4250–4254 (1994).
Levy, A. B. Aromatic substitution via organoboranes. 3. Simultaneous regiospecific functionalization of the indole nucleus at the 2- and 3-positions. Tetrahedron Lett. 20, 4021–4024 (1979).
Brown, H. C., De Lue, N. R., Kabalka, G. W. & Hedgecock, H. C. J. Consistent inversion in the base-induced reaction of iodine with organoboranes. A convenient procedure for the synthesis of optically active iodides. J. Am. Chem. Soc. 98, 1290–1291 (1976).
Kabalka, G. W. & Gooch, E. E. I. A mild and convenient procedure for conversion of alkenes into alkyl iodides via reaction of iodine monochloride with organoboranes. J. Org. Chem. 45, 3578–3580 (1980).
Brown, H. C. & Lane, C. F. Base-induced bromination of tri-exo-norbornylborane: an electrophilic substitution with predominant inversion of configuration. J. Chem. Soc. Chem. Commun. 521–522 (1971).
Larouche-Gauthier, R., Elford, T. G. & Aggarwal, V. K. Ate complexes of secondary boronic esters as chiral organometallic-type nucleophiles for asymmetric synthesis. J. Am. Chem. Soc. 133, 16794–16797 (2011).
Stymiest, J. L., Dutheuil, D., Mohmood, E. & Aggarwal, V. K. Lithiated carbamates: chiral carbenoids for iterative homologation of boranes and boronic esters. Angew. Chem. Int. Ed. 46, 7491–7494 (2007).
Stymiest, J. L., Bagutski, V., French, R. M. & Aggarwal, V. K. Enantiodivergent conversion of chiral secondary alcohols into tertiary alcohols. Nature 456, 778–782 (2008).
Bagutski, V., French, R. M. & Aggarwal, V. K. Full chirality transfer in the conversion of secondary alcohols into tertiary boronic esters and alcohols using lithiation–borylation reactions. Angew. Chem. Int. Ed. 49, 5142–5145 (2010).
Pulis, A. P., Blair, D. J., Torres, E. & Aggarwal, V. K. Synthesis of enantioenriched tertiary boronic esters by the lithiation/borylation of secondary alkyl benzoates. J. Am. Chem. Soc. 135, 16054–16057 (2013).
Batsanov, A. S., Grosjean, C., Schultz, T. & Whiting, A. A (–)-Sparteine-directed highly enantioselective synthesis of boroproline. Solid- and solution-state structure and properties. J. Org. Chem. 72, 6276–6279 (2007).
Villa, G., Povie, G. & Renaud, P. Radical chain reduction of alkylboron compounds with catechols. J. Am. Chem. Soc. 133, 5913–5920 (2011).
Paras, N., Simmons, B. & MacMillan, D. W. C. A process for the rapid removal of dialkylamino-substituents from aromatic rings. Application to the expedient synthesis of (R)-tolterodine. Tetrahedron 65, 3232–3238 (2009).
Blakey, S. B. & MacMillan, D. W. C. The first Suzuki cross-coupling of aryltrimethylammonium ions. J. Am. Chem. Soc. 125, 6046–6047 (2003).
Koehn, F. E. & Carter, G. T. The evolving role of natural products in drug discovery. Nature Rev. 4, 206–220 (2005).
Mohiti, M., Rampalakos, C., Feeney, K., Leonori, D. & Aggarwal, V. K. Asymmetric addition of chiral boron-ate complexes to cyclic iminium ions. Chem. Sci. 5, 602–607 (2013).
Berionni, G., Maji, B., Knochel, P. & Mayr, H. Nucleophilicity parameters for designing transition metal-free C–C bond forming reactions of organoboron compounds. Chem. Sci. 3, 878–882 (2012).
Reichardt, C. & Welton, T. E. Solvents and Solvent Effects in Organic Chemistry 4th edn (Wiley, 2010).
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EP/I038071/1) and the European Research Council (FP7/2007-2013, ERC grant no. 246785) for financial support. A.B. thanks the Marie Curie Fellowship program (EC FP7 No 329578). We thank H. Mayr, V. Rawal and J. N. Harvey for useful discussions.
Author information
Authors and Affiliations
Contributions
D.L. and V.K.A. designed the project and wrote the manuscript. A.B. and M.O. contributed intellectually and practically to the laboratory experiments, S.E. performed preliminary computational studies and was involved in the mechanistic discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 8606 kb)
Rights and permissions
About this article
Cite this article
Bonet, A., Odachowski, M., Leonori, D. et al. Enantiospecific sp2–sp3 coupling of secondary and tertiary boronic esters. Nature Chem 6, 584–589 (2014). https://doi.org/10.1038/nchem.1971
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1971
This article is cited by
-
Enantioselective C–C cross-coupling of unactivated alkenes
Nature Catalysis (2023)
-
Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A
Nature Chemistry (2023)
-
Photo-induced trifunctionalization of bromostyrenes via remote radical migration reactions of tetracoordinate boron species
Nature Communications (2022)
-
Stereoselective hydrogen atom transfer to acyclic radicals: a switch enabling diastereodivergent borylative radical cascades
Nature Communications (2022)
-
A radical approach for the selective C–H borylation of azines
Nature (2021)