Abstract
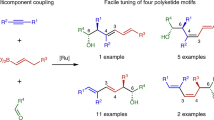
Multicomponent reactions allow for more bond-forming events per synthetic operation, enabling more step- and time-economical conversion of simple starting materials to complex and thus value-added targets. These processes invariably require that reactivity be relayed from intermediate to intermediate over several mechanistic steps until a termination event produces the final product. Here, we report a multicomponent process in which a novel 1,2,3-butatriene equivalent (TMSBO: TMSCH2C≡CCH2OH) engages chemospecifically as a two-carbon alkyne component in a metal-catalysed [5 + 2] cycloaddition with a vinylcyclopropane to produce an intermediate cycloadduct. Under the reaction conditions, this intermediate undergoes a remarkably rapid 1,4-Peterson elimination, producing a reactive four-carbon diene intermediate that is readily intercepted in either a metal-catalysed or thermal [4 + 2] cycloaddition. TMSBO thus serves as an yne-to-diene transmissive reagent coupling two powerful and convergent cycloadditions—the homologous Diels–Alder and Diels–Alder cycloadditions—through a vinylogous Peterson elimination, and enabling flexible access to diverse polycycles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wender, P. A. et al. Toward the ideal synthesis. New transition metal-catalyzed reactions inspired by novel medicinal leads. Pure Appl. Chem. 74, 25–31 (2002).
Wender, P. A. & Miller, B. L. Synthesis at the molecular frontier. Nature 460, 197–201 (2009).
Wender, P. A. & Miller, B. L. in Organic Synthesis: Theory and Applications (ed. Hudlicky, T.) 27–66 (JAI Press, 1993).
Tietze, L. F. & Modi, A. Multicomponent domino reactions for the synthesis of biologically active natural products and drugs. Med. Res. Rev. 20, 304–322 (2000).
Clavier, H. & Pellissier, H. Recent developments in enantioselective metal-catalyzed domino reactions. Adv. Synth. Catal. 354, 3347–3403 (2012).
Slobbe, P., Ruijter, E. & Orru, R. V. A. Recent applications of multicomponent reactions in medicinal chemistry. Med. Chem. Commun. 3, 1189–1218 (2012).
Kang, T., Kim, W-Y., Yoon, Y., Kim, B. G. & Lee, H-Y. Tandem cycloaddition reactions of allenyl diazo compounds forming triquinanes via trimethylenemethane diyls. J. Am. Chem. Soc. 133, 18050–18053 (2011).
Van der Heijden, G., Ruijter, E. & Orru, R. V. A. Efficiency, diversity, and complexity with multicomponent reactions. Synlett 24, 666–685 (2013).
Hopf, H. & Sherburn, M. S. Dendralenes branch out: cross-conjugated oligoenes allow the rapid generation of molecular complexity. Angew. Chem. Int. Ed. 51, 2298–2338 (2012).
Souweha, M. S., Enright, G. D. & Fallis, A. G. Vinigrol: a compact, diene-transmissive Diels–Alder strategy to the tricyclic core. Org. Lett. 9, 5163–5166 (2007).
Gani, O. A. B. S. M. & Engh, R. A. Protein kinase inhibition of clinically important staurosporine analogues. Nat. Prod. Rep. 27, 489–498 (2010).
Awuah, E. & Capretta, A. Development of methods for the synthesis of libraries of substituted maleimides and α,β-unsaturated-γ-butyrolactams. J. Org. Chem. 76, 3122–3130 (2011).
Tanramluk, D., Schreyer, A., Pitt, W. R. & Blundell, T. L. On the origins of enzyme inhibitor selectivity and promiscuity: a case study of protein kinase binding to staurosporine. Chem. Biol. Drug Des. 74, 16–24 (2009).
Chae, H. J. et al. Molecular mechanism of staurosporine-induced apoptosis in osteoblasts. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 42, 373–381 (2000).
Omura, S. et al. A new alkaloid AM-2282 of Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. (Tokyo) 30, 275–282 (1977).
Angus, R. O. Jr & Johnson, R. P. Butatriene cycloaddition equivalent approach to the multiple linear homologation of six-membered rings and the synthesis of benzocyclobutenes. J. Org. Chem. 48, 273–276 (1983).
Hojo, M., Tomita, K., Hirohara, Y. & Hosomi, A. New access to di-exo-methylenecyclobutanes via [2+2] cycloaddition of 3-methylthio-4-trimethylsilyl-1,2-butadiene with alkenes mediated by a Lewis acid. Tetrahedron Lett. 34, 8123–8126 (1993).
Hojo, M., Murakami, C., Nakamura, S. & Hosomi, A. Allenylmethylsilane derivative as a synthetic equivalent of 1,2,3-butatriene: synthesis and reactions of di-exo-methylenecyclobutanes and -cyclobutenes. Chem. Lett. 27, 331–332 (1998).
Hojo, M., Tomita, K. & Hosomi, A. Synthesis and reactions of methyl (trimethylsilylmethyl)-acetylenecarboxylate. A general method for the generation of di-exo-methyleneisoxazolines and novel access to fused isoxazoles. Tetrahedron Lett. 34, 485–488 (1993).
Leroyer, L., Maraval, V. & Chauvin, R. Synthesis of the butatriene C4 function: methodology and applications. Chem. Rev. 112, 1310–1343 (2012).
Schubert, W. M., Liddicoet, T. H. & Lanka, W. A. The synthesis of butatriene. J. Am. Chem. Soc. 74, 569–569 (1952).
Chow, H-F., Cao, X-P. & Leung, M. Facile synthesis of alkyl and aryl substituted 1,2,3-butatrienes. J. Chem. Soc. Chem. Commun. 2121–2122 (1994).
Wang, K. K., Liu, B. & Lu, Y. Facile synthesis of [3]cumulenes via 1,4-elimination of hydroxytrimethylsilane from 4-(trimethylsilyl)-2-butyn-1-ols. J. Org. Chem. 60, 1885–1887 (1995).
Malkov, A. V. et al. A novel bifunctional allyldisilane as a triple allylation reagent in the stereoselective synthesis of trisubstituted tetrahydrofurans. Chem. Eur. J. 17, 7162–7166 (2011).
Jervis, P. J., Kariuki, B. M. & Cox, L. R. Stereoselective synthesis of 2,4,5-trisubstituted tetrahydropyrans using an intramolecular allylation strategy. Org. Lett. 8, 4649–4652 (2006).
Chiu, S. K. & Peterson, P. E. The preparation of propargyltrimethylsilanes. Tetrahedron Lett. 21, 4047–4050 (1980).
Tong, R. et al. Total syntheses of durgamone, nakorone, and abudinol B via biomimetic oxa- and carbacyclizations. J. Am. Chem. Soc. 129, 1050–1051 (2007).
Ambasht, S., Chiu, S. K., Peterson, P. E. & Queen, J. Preparation of trimethylsilylmethyl derivatives using phase transfer methods. Synthesis 1980, 318–320 (1980).
Wein, A. N., Tong, R. & McDonald, F. E. An economical synthesis of 4-trimethylsilyl-2-butyn-1-ol. Org. Synth. 88, 296–308 (2011).
Fleming, I., Morgan, I. T. & Sarkar, A. K. The stereochemistry of the vinylogous Peterson elimination. J. Chem. Soc. Perkin Trans. 1 2749–2764 (1998).
Harmata, M. & Bohnert, G. J. A 4+3 cycloaddition approach to the synthesis of (±)-sterpurene. Org. Lett. 5, 59–61 (2003).
Angell, R., Parsons, P. J., Naylor, A. & Tyrrell, E. An extremely mild method for the construction of E-1,3-dienes. Synlett 1992, 599–600 (1992).
Takano, S., Otaki, S. & Ogasawara, K. A Facile synthesis of the substituted tetrahydronapthalenes by the benzo-Peterson reaction. Heterocycles 23, 2811–2814 (1985).
Takano, S., Sato, N., Otaki, S. & Ogasawara, K. The total synthesis of (±)-sikkimotoxin via the benzo-Peterson reaction. Heterocycles 25, 69–73 (1987).
Takano, S., Otaki, S. & Ogasawara, K. Stereocontrolled synthesis of (±)-deoxypodophyllotoxin via the benzyl equivalent of the Peterson reaction. J. Chem. Soc. Chem. Commun. 485–487 (1985).
Angoh, A. G. & Clive, D. L. J. A synthetic equivalent for the butadienyl carbonium ion: use of 4-(trimethylsilyl)but-2-ynal for preparation of 1,3-dienes and macroexpansion of cyclic ketones. J. Chem. Soc. Chem. Commun. 534–536 (1984).
Ahmed, M., Atkinson, C. E., Barrett, A. G. M., Malagu, K. & Procopiou, P. A. Synthesis of C-19-functionalized 1α-hydroxyvitamin D2 analogues via ring-closing metathesis. Org. Lett. 5, 669–672 (2003).
Wender, P. A. & Williams, T. J. [(arene)Rh(cod)]+ complexes as catalysts for [5+2] cycloaddition reactions. Angew. Chem. Int. Ed. 41, 4550–4553 (2002).
Acknowledgements
This research was supported by the National Science Foundation (NSF, CHE1265956) and the National Institutes of Health (CA031841). Additional funding was provided by the NSF Graduate Research Fellowship (R.V.Q.), an Abbott Laboratories Stanford Graduate Fellowship (M.S.J.), Kanazawa University (F.I.) and the German Academic Exchange Service (M.P.).
Author information
Authors and Affiliations
Contributions
P.A.W. conceived the study. F.I. and M.P. performed the initial syntheses of butynols and evaluated the initial viability of [5 + 2] and [5 + 2]/[4 + 2] reactions. D.N.F., M.S.J. and R.V.Q. determined the substrate profile and performed the synthesis and characterization of all reported compounds. M.S.J., R.V.Q. and P.A.W. wrote the paper. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2565 kb)
Rights and permissions
About this article
Cite this article
Wender, P., Fournogerakis, D., Jeffreys, M. et al. Structural complexity through multicomponent cycloaddition cascades enabled by dual-purpose, reactivity regenerating 1,2,3-triene equivalents. Nature Chem 6, 448–452 (2014). https://doi.org/10.1038/nchem.1917
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1917
This article is cited by
-
An enantioselective four-component reaction via assembling two reaction intermediates
Nature Communications (2022)
-
Computational ligand design in enantio- and diastereoselective ynamide [5+2] cycloisomerization
Nature Communications (2016)