Abstract
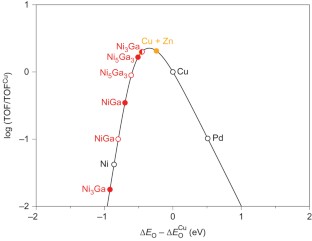
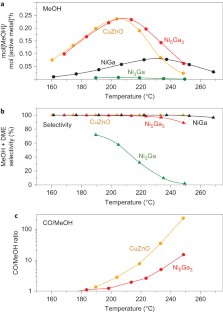
The use of methanol as a fuel and chemical feedstock could become very important in the development of a more sustainable society if methanol could be efficiently obtained from the direct reduction of CO2 using solar-generated hydrogen. If hydrogen production is to be decentralized, small-scale CO2 reduction devices are required that operate at low pressures. Here, we report the discovery of a Ni-Ga catalyst that reduces CO2 to methanol at ambient pressure. The catalyst was identified through a descriptor-based analysis of the process and the use of computational methods to identify Ni-Ga intermetallic compounds as stable candidates with good activity. We synthesized and tested a series of catalysts and found that Ni5Ga3 is particularly active and selective. Comparison with conventional Cu/ZnO/Al2O3 catalysts revealed the same or better methanol synthesis activity, as well as considerably lower production of CO. We suggest that this is a first step towards the development of small-scale low-pressure devices for CO2 reduction to methanol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schlögl, R. Chemistry's role in regenerative energy. Angew. Chem. Int. Ed. 50, 6424–6426 (2011).
Olah, G. A. Towards oil independence through renewable methanol chemistry. Angew. Chem. Int. Ed. 52, 104–107 (2013).
Benson, E. E., Kubiak, C. P., Sathrum, A. J. & Smieja, J. M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 38, 89–99 (2009).
Arakawa, H. et al. Catalysis research of relevance to carbon management: progress, challenges, and opportunities. Chem. Rev. 101, 953–996 (2001).
Hori, Y., Kikuchi, K. & Suzuki, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrocarbonate solution. Chem. Lett. 14, 1695–1698 (1985).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Rosen, B. A. et al. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 334, 643–644 (2011).
Cole, E. B. et al. Using a one-electron shuttle for the multielectron reduction of CO2 to methanol: kinetic, mechanistic, and structural insights. J. Am. Chem. Soc. 132, 11539–11551 (2010).
Schouten, K. J. P., Kwon, Y., van der Ham, C. J. M., Qin, Z. & Koper, M. T. M. A new mechanism for the selectivity to C(1) and C(2) species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem. Sci. 2, 1902–1909 (2011).
Chen, Y., Li, C. W. & Kanan, M. W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 134, 19969–19972 (2012).
Peterson, A. A. & Nørskov, J. K. Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts. J. Phys. Chem. Lett. 3, 251–258 (2012).
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006).
Crabtree, G. & Sarrao, J. The road to sustainability. Physics World 22, 24–30 (2009).
Hansen, J. B. & Nielsen, P. E. H. in Handbook of Heterogeneous Catalysis (eds Ertl, G., Knözinger, H. & Schüth, F.) 2920 (Wiley, 2008).
Kasatkin, I., Kurr, P., Kniep, B., Trunschke, A. & Schlögl, R. Role of lattice strain and defects in copper particles on the activity of Cu/ZnO/Al2O3 catalysts for methanol synthesis. Angew. Chem. Int. Ed. 46, 7324–7327 (2007).
Behrens, M. Meso- and nano-structuring of industrial Cu/ZnO/(Al2O3) catalysts. J. Catal. 267, 24–29 (2009).
Kurtz, M., Wilmer, H., Genger, T., Hinrichsen, O. & Muhler, M. Deactivation of supported copper catalysts for methanol synthesis. Catal. Lett. 86, 77–80 (2003).
Campbell, C. T., Daube, K. A. & White, J. M. Cu/ZnO(0001) and ZnOx/Cu(111): model catalysts for methanol synthesis. Surf. Sci. 182, 458–476 (1987).
Bowker, M., Hadden, R. A., Houghton, H., Hyland, J. N. K. & Waugh, K. C. The mechanism of methanol synthesis on copper/zinc oxide/alumina catalysts. J. Catal. 109, 263–273 (1988).
Askgaard, T. S., Nørskov, J. K., Ovesen, C. V. & Stoltze, P. A kintic model of methanol synthesis. J. Catal. 156, 229–242 (1995).
Fisher, I. A. & Bell, A. T. In-situ infrared study of methanol synthesis from H2/CO2 over Cu/SiO2 and Cu/ZrO2/SiO2 . J. Catal. 172, 222–237 (1997).
Meitzner, G. & Iglesia, E. New insights into methanol synthesis catalysts from X-ray absorption spectroscopy. Catal. Today 53, 433–441 (1999).
Fujitani, T. & Nakamura, J. The chemical modification seen in the Cu/ZnO methanol synthesis catalysts. Appl. Catal. A 191, 111–129 (2000).
Grunwaldt, J-D., Molenbroek, A. M., Topsøe, N-Y., Topsøe, H. & Clausen, B. S. In situ investigations of structural changes in Cu/ZnO catalysts. J. Catal. 194, 452–460 (2000).
Hansen, P. L. et al. Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science 295, 2053–2055 (2002).
Kurtz, M. et al. New synthetic routes to more active Cu/ZnO catalysts used for methanol synthesis. Catal. Lett. 92, 49–52 (2004).
Waugh, K. C. Methanol synthesis. Catal. Lett. 142, 1153–1166 (2012).
Yang, Y., Mims, C. A., Mei, D. H., Peden, C. H. F. & Campbell, C. T. Mechanistic studies of methanol synthesis over Cu from CO/CO2/H2/H2O mixtures: the source of C in methanol and the role of water. J. Catal. 298, 10–17 (2013).
Grabow, L. C. & Mavrikakis, M. Mechanism of methanol synthesis on Cu through CO2 and CO hydrogenation. ACS Catal. 1, 365–384 (2011).
Behrens, M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012).
Grabow, L. C. et al. Descriptor-based analysis applied to HCN synthesis from NH3 and CH4 . Angew. Chem. Int. Ed. 50, 4601–4605 (2011).
Lausche, A. C., Hummelshøj, J. S., Abild-Pedersen, F., Studt, F. & Nørskov, J. K. Application of a new informatics tool in heterogeneous catalysis: analysis of methanol dehydrogenation on transition metal catalysts for the production of anhydrous formaldehyde. J. Catal. 291, 133–137 (2012).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Nørskov, J. K. et al. The nature of the active site in heterogeneous metal catalysis. Chem. Soc. Rev. 37, 2163–2171 (2008).
Studt, F., Abild-Pedersen, F., Varley, J. B. & Nørskov, J. K. CO and CO2 hydrogenation to methanol calculated using the BEEF–vdW functional. Catal. Lett. 143, 71–73 (2013).
Wellendorff, J. et al. Density functionals for surface science: exchange-correlation model development with Bayesian error estimation. Phys. Rev. B 85, 235149 (2012).
Nørskov, J. K., Abild-Pedersen, F., Studt, F. & Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl Acad. Sci. USA 108, 937–943 (2011).
Studt, F. et al. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 320, 1320–1322 (2008).
Okamoto, H. Ga-Ni (gallium-nickel). J. Phase Equilib. Diffus. 31, 575–576 (2010).
Baltes, C., Vukojević, S. & Schüth, F. Correlations between synthesis, precursor, and catalyst structure and activity of a large set of CuO/ZnO/Al2O3 catalysts for methanol synthesis. J. Catal. 258, 334–344 (2008).
Arico, A. S., Srinivasan, S. & Antonucci, V. DMFCs: from fundamental aspects to technology development. Fuel Cells 1, 133–161 (2001).
Kamarudin, S. K., Daud, W. R. W., Ho, S. L. & Hasran, U. A. Overview on the challenges and developments of micro-direct methanol fuel cells (DMFC). J. Power Sources 163, 743–754 (2007).
Lackner, K. S. A guide to CO2 sequestration. Science 300, 1677–1678 (2003).
Keith, D. W. Why capture CO2 from the atmosphere? Science 325, 1654–1655 (2009).
Jones, C. W. CO2 capture from dilute gases as a component of modern global carbon management. Annu. Rev. Chem. Biomol. Eng. 2, 31–52 (2011).
House, K. Z. et al. Economic and energetic analysis of capturing CO2 from ambient air. Proc. Natl Acad. Sci. USA 108, 20428–20433 (2011).
Studt, F. et al. CO hydrogenation to methanol on Cu-Ni catalysts: theory and experiment. J. Catal. 293, 51–60 (2012).
Hummelshøj, J. S., Abild-Pedersen, F., Studt, F., Bligaard, T. & Nørskov, J. K. CatApp: a web application for surface chemistry and heterogeneous catalysis. Angew. Chem. Int. Ed. 51, 272–274 (2012).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Acknowledgements
F.S., F.A-P., J.S.H. and J.K.N. acknowledge support from the US Department of Energy. This work was partly supported by The Danish National Research Foundation's Centre for Individual Nanoparticle Functionality (DNRF54) and partly by the Catalysis for Sustainable Energy initiative, which is funded by the Danish Ministry of Science, Technology, and Innovation. The authors also thank J. R. Rostrup-Nielsen for helpful discussions.
Author information
Authors and Affiliations
Contributions
F.S., F.A-P., J.S.H. and J.K.N. contributed to the computational work in this article. I.S., C.F.E., S.D. and I.C. contributed to the experimental work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2711 kb)
Rights and permissions
About this article
Cite this article
Studt, F., Sharafutdinov, I., Abild-Pedersen, F. et al. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nature Chem 6, 320–324 (2014). https://doi.org/10.1038/nchem.1873
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1873
This article is cited by
-
Upgrading CO2 to sustainable aromatics via perovskite-mediated tandem catalysis
Nature Communications (2024)
-
Homolytic H2 dissociation for enhanced hydrogenation catalysis on oxides
Nature Communications (2024)
-
Assignment of individual structures from intermetalloid nickel gallium cluster ensembles
Communications Chemistry (2024)
-
Dynamic configurations of metallic atoms in the liquid state for selective propylene synthesis
Nature Nanotechnology (2023)
-
Strained few-layer MoS2 with atomic copper and selectively exposed in-plane sulfur vacancies for CO2 hydrogenation to methanol
Nature Communications (2023)