Abstract
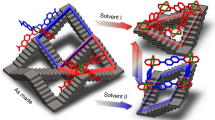
Porous materials are attractive for separation and catalysis—these applications rely on selective interactions between host materials and guests. In metal–organic frameworks (MOFs), these interactions can be controlled through a flexible structural response to the presence of guests. Here we report a MOF that consists of glycyl–serine dipeptides coordinated to metal centres, and has a structure that evolves from a solvated porous state to a desolvated non-porous state as a result of ordered cooperative, displacive and conformational changes of the peptide. This behaviour is driven by hydrogen bonding that involves the side-chain hydroxyl groups of the serine. A similar cooperative closure (reminiscent of the folding of proteins) is also displayed with multipeptide solid solutions. For these, the combination of different sequences of amino acids controls the framework's response to the presence of guests in a nonlinear way. This functional control can be compared to the effect of single-point mutations in proteins, in which exchange of single amino acids can radically alter structure and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zhou, H-C., Long, J. R. & Yaghi, O. M. Introduction to metal–organic frameworks. Chem. Rev. 112, 673–674 (2012).
Li, J. R., Kuppler, R. J. & Zhou, H. C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 38, 1477–1504 (2009).
Yang, W. et al. Selective CO2 uptake and inverse CO2/C2H2 selectivity in a dynamic bifunctional metal–organic framework. Chem. Sci. 3, 2993–2999 (2012).
Dhakshinamoorthy, A. & García, H. Catalysis by metal nanoparticles embedded on metal–organic frameworks. Chem. Soc. Rev. 41, 5262–5284 (2012).
Lee, J-Y. et al. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 38, 1450–1459 (2009).
Rocca, D. J., Liu, D. & Lin, W. Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 44, 957–968 (2011).
Bauer, C. A. et al. Influence of connectivity and porosity on ligand-based luminescence in zinc metal–organic frameworks. J. Am. Chem. Soc. 129, 7136–7144 (2007).
Ferrando-Soria, J. et al. Highly selective chemical sensing in a luminescent nanoporous magnet. Adv. Mater. 24, 5625–5629 (2012).
Deng, H. et al. Large-pore apertures in a series of metal–organic frameworks. Science 336, 1018–1023 (2012).
Guillerm, V. et al. A series of isoreticular, highly stable, porous zirconium oxide based metal–organic frameworks. Angew. Chem. Int. Ed. 51, 9267–9271 (2012).
Hasegawa, S. et al. Three-dimensional porous coordination polymer functionalized with amide groups based on tridentate ligand: selective sorption and catalysis. J. Am. Chem. Soc. 129, 2607–2614 (2007).
Barrio, J. et al. Control of porosity geometry in amino acid derived nanoporous materials. Chem. Eur. J. 14, 4521–4532 (2008).
An, J., Geib, S. J. & Rosi, N. L. High and selective CO2 uptake in a cobalt adeninate metal–organic framework exhibiting pyrimidine- and amino-decorated pores. J. Am. Chem. Soc. 132, 38–39 (2009).
Imaz, I. et al. Metal–biomolecule frameworks (MBioFs). Chem. Commun. 47, 7287–7302 (2011).
Boehr, D. D., Nussinov, R. & Wright, P. E. The role of dynamic conformational ensembles in biomolecular recognition. Nature Chem. Biol. 5, 789–796 (2009).
Rabone, J. et al. An adaptable peptide-based porous material. Science 329, 1053–1057 (2010).
Martí-Gastaldo, C., Warren, J. E., Stylianou, K., Flack, N. L. O. & Rosseinsky, M. J. Enhanced stability in rigid peptide-based porous materials. Angew. Chem. Int. Ed. 51, 11044–11048 (2012).
Connolly, M. L. Solvent-accessible surfaces of proteins and nucleic acids. Science 221, 709–713 (1983).
Deng, H. et al. Multiple functional groups of varying ratios in metal–organic frameworks. Science 327, 846–850 (2010).
Fukushima, T. et al. Solid solutions of soft porous coordination polymers: fine-tuning of gas adsorption properties. Angew. Chem. Int. Ed. 49, 4820–4824 (2010).
Burrows, A. D. Mixed-component metal–organic frameworks (MC-MOFs): enhancing functionality through solid solution formation and surface modifications. CrystEngComm 13, 3623–3642 (2011).
Henke, S., Schneemann, A., Wütscher, A. & Fischer, R. A. Directing the breathing behavior of pillared-layered metal–organic frameworks via a systematic library of functionalized linkers bearing flexible substituents. J. Am. Chem. Soc. 134, 9464–9474 (2012).
Lescouet, T. et al. Homogeneity of flexible metal–organic frameworks containing mixed linkers. J. Mater. Chem. 22, 10287–10293 (2012).
Serra-Crespo, P. et al. Interplay of metal node and amine functionality in NH2-MIL-53: modulating breathing behavior through intra-framework interactions. Langmuir 28, 12916–12922 (2012).
Maes, M. et al. Separation of styrene and ethylbenzene on metal–organic frameworks: analogous structures with different adsorption mechanisms. J. Am. Chem. Soc. 132, 15277–15285 (2010).
Biradha, K., Hongo, Y. & Fujita, M. Crystal-to-crystal sliding of 2D coordination layers triggered by guest exchange. Angew. Chem. Int. Ed. 41, 3395–3398 (2002).
Kitaura, R., Seki, K., Akiyama, G. & Kitagawa, S. Porous coordination-polymer crystals with gated channels specific for supercritical gases. Angew. Chem. Int. Ed. 42, 428–431 (2003).
Li, D. & Kaneko, K. Hydrogen bond-regulated microporous nature of copper complex-assembled microcrystals. Chem. Phys. Lett. 335, 50–56 (2001).
Maspoch, D. et al. A nanoporous molecular magnet with reversible solvent-induced mechanical and magnetic properties. Nature Mater. 2, 190–195 (2003).
Lu, J. Y. & Babb, A. M. An extremely stable open-framework metal–organic polymer with expandable structure and selective adsorption capability. Chem. Commun. 1340–1341 (2002).
Choi, H. J., Dincă, M. & Long, J. R. Broadly hysteretic H2 adsorption in the microporous metal–organic framework Co(1,4-benzenedipyrazolate). J. Am. Chem. Soc. 130, 7848–7850 (2008).
Salles, F. et al. Multistep N2 breathing in the metal–organic framework Co(1,4-benzenedipyrazolate). J. Am. Chem. Soc. 132, 13782–13788 (2010).
Horcajada, P. et al. How linker's modification controls swelling properties of highly flexible iron(III) dicarboxylates MIL-88. J. Am. Chem. Soc. 133, 17839–17847 (2011).
Aguado, S. et al. Guest-induced gate-opening of a zeolite imidazolate framework. New J. Chem. 35, 546–550 (2011).
Hu, J-S. et al. Syntheses, structures, and photoluminescence of five new metal–organic frameworks based on flexible tetrapyridines and aromatic polycarboxylate acids. Cryst. Growth Des. 10, 2676–2684 (2010).
Colombo, V. et al. Tuning the adsorption properties of isoreticular pyrazolate-based metal–organic frameworks through ligand modification. J. Am. Chem. Soc. 134, 12830–12843 (2012).
Henke, S., Schmid, R., Grunwaldt, J-D. & Fischer, R. A. Flexibility and sorption selectivity in rigid metal–organic frameworks: the impact of ether-functionalised linkers. Chem. Eur. J. 16, 14296–14306 (2010).
Kepert, C. J., Prior, T. J. & Rosseinsky, M. J. A versatile family of interconvertible microporous chiral molecular frameworks: the first example of ligand control of network chirality. J. Am. Chem. Soc. 122, 5158–5168 (2000).
Czepirski, L. & Jagiello, J. Virial-type thermal equation of gas solid adsorption. Chem. Eng. Sci. 44, 797–801 (1989).
Klafter, J. & Shlesinger, M. F. On the relationship among three theories of relaxation in disordered systems. Proc. Natl Acad. Sci. USA 83, 848–851 (1986).
Mittermaier, A. New tools provide new insights in NMR studies of protein dynamics. Science 312, 224–228 (2006).
Teague, S. J. Implications of protein flexibility for drug discovery. Nature Rev. Drug Discov. 2, 527–541 (2003).
French-Constant, R. H., Rocheleau, T. A., Steichen, J. C. & Chalmers, A. E. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature 363, 449–451 (1993).
Altschmied, L., Baumeister, R., Pfleiderer, K. & Hillen, W. A threonine to alanine exchange at position 40 of Tet repressor alters the recognition of the sixth base pair of tet operator from GC to AT. EMBO J. 7, 4011–4017 (1988).
Acknowledgements
Research was supported by the Engineering and Physical Sciences Research Council (EPSRC) under EP/H000925 and EP/J008834. C.M.G. thanks the European Union for a Marie Curie Fellowship (IEF-253369). Via our membership of the UK's high-performance computing Materials Chemistry Consortium, funded by EPSRC (EP/F067496), this work made use of the facilities of HECToR, the UK's national high-performance computing service. We also thank the support of the Diamond Light Source and Anna Warren.
Author information
Authors and Affiliations
Contributions
C.M.G and M.J.R conceived the research, designed the experiments and co-wrote the paper. C.M.G synthesized the materials and carried out the adsorption experiments and their analysis. D.A. designed and performed the theoretical simulations under the guidance of G.R.D., and N.G.B., J.E.W., P.A.C. and G.M. performed the structural analyses. Y.Z.K. and P.V.W. carried out and analysed the solid-state CP/MAS NMR experiments. M.E.B. performed NMR spectroscopy on multiple-peptide MOFs. All the authors discussed the results and contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 9788 kb)
Supplementary information
Crystallographic data for ZnGS2 (sntB00014C1). (CIF 283 kb)
Supplementary information
Crystallographic data for Zn[(GA)0.5(GS)0.5]2 (B00113). (CIF 391 kb)
Supplementary information
Crystallographic data for Zn[(GA)0.5(GT)0.5]2 (B00122). (CIF 404 kb)
Supplementary information
Crystallographic data for compound Zn[(GS)0.5(GT)0.5]2 (B00117). (CIF 204 kb)
Supplementary information
Crystallographic data for compound Zn[(GS)0.75(GT)0.25]2 (MJR0981). (CIF 17 kb)
Supplementary information
Crystallographic data for compound desolvated Zn[(GS)0.75(GT)0.25]2 (MJR0981post). (CIF 16 kb)
Rights and permissions
About this article
Cite this article
Martí-Gastaldo, C., Antypov, D., Warren, J. et al. Side-chain control of porosity closure in single- and multiple-peptide-based porous materials by cooperative folding. Nature Chem 6, 343–351 (2014). https://doi.org/10.1038/nchem.1871
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1871
This article is cited by
-
Mesoporous Cu3−xZnx(BTC)2 nanocubes synthesized in deep eutectic solvent and their catalytic performances
Nano Research (2023)
-
Peptide-derived coordination frameworks for biomimetic and selective separation
Analytical and Bioanalytical Chemistry (2023)
-
Peptide-based porous materials and their applications
Science China Materials (2023)
-
Porous and permutable peptide frameworks
Nature Chemistry (2022)
-
Stimuli-responsive metal–organic frameworks enabled by intrinsic molecular motion
Nature Materials (2022)